How do healthy cells defend themselves against a virus?
New study with Italian colleagues reveals a pivotal moment in anti-viral defense
Briefs
In the fight against viral infection, our immune system is very much like a tree: It branches out in different directions, adopting different defensive strategies depending on the root of the problem. New research by the Weizmann Institute’s Prof. Ido Amit and Prof. Matteo Iannacone San Raffaele Hospital in Milan reveals a “branching” mechanism governed by interferon—a protein that issues instructions that prepare cells to mount an anti-viral defense.
According to the new study, published in Nature Immunology in February, the timing of interferon-based messages is significant, determining which of two distinct cell types will be generated by the immune system. Clarifying an important dynamic that regulates the adaptive immune response—the response in which the body generates pathogen-fighting antibodies—these findings could help pharmaceutical companies design more effective vaccines.
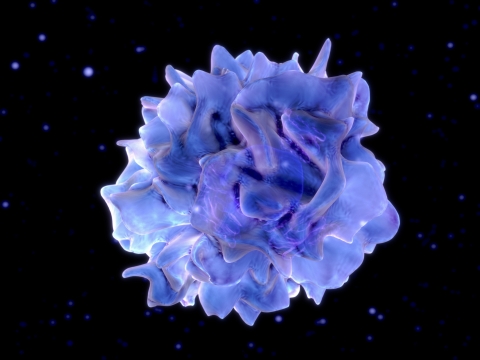
A dendritic cell
The study, performed by Prof. Amit and the postdoctoral fellow, Dr. Amir Giladi, of the Department of Immunology together with Prof. Matteo Iannacone and other colleagues from Italy and Germany, focused on so-called “helper” T cells. These immune agents do not neutralize invaders themselves, but rather send out messages that trigger the body’s differential immune response, allowing our cells to make critical choices at a critical time.
Branching dynamic
The scientists identified a “branching” dynamic—think family tree—that drives the production of two different classes of helper T cells. Based on the messages produced by interferon—the protein that triggers a cellular response to infection—the final determination of the type of helper T cells generated depends on when these messages are received.
During viral infection, interferon’s “warning messages” are delivered to a specific address: precursor cells of the immune system known as dendritic cells (DCs). If the message is delivered early on in the infection process—as in the case of the first virus that the scientists studied—the message triggers the formation of helper T cells of a specific type. When the messages reach the DCs later on—as occurs during infection by the second virus—the result is a different type of helper T cell altogether.
The researchers used single cell RNA-sequencing, a novel technology that Prof. Amit’s lab has pioneered, which allows high-resolution concurrent profiling of a massive number of immune cells, to profile and molecularly define the participating immune cells at different time points of infection. Using this technology, they could identify the exact nature of DCs that received the interferon signal and the precise timing of the ensuing events. This revealed that, depending on the virus and its timing, DCs use different signaling molecules to cue T cells toward specific helper types.
Fittingly, the term dendritic is derived from dendron—Greek for tree—aptly illustrating how the immune response, rooted in a particular type of virus, branches out to mount the most effective defense possible. Thanks to the work of Prof. Amit and Prof. Matteo Iannacone and colleagues, vaccine designers may someday be able to build this molecular dynamic into their clinical formulations, providing better defense against viral infection.
Prof. Ido Amit is supported by the Helen and Martin Kimmel Award for Innovative Investigation, the Sagol Institute for Longevity Research, the Kekst Family Institute for Medical Genetics, the Thompson Family Foundation Alzheimer's Research Fund, Foundation Adelis, the Steven B. Rubenstein Research Fund for Leukemia and Other Blood Disorders, the Garvan Weizmann Partnership, Rising Tide Foundation, Anita James Rosen Foundation, Wolfson Family Charitable Trust, Estate of Simon Saretzky, and the Estate of Arthur Rath. He is the incumbent of the Eden and Steven Romick Professorial Chair.