The role of PCNA ubiquitination in translesion DNA synthesis
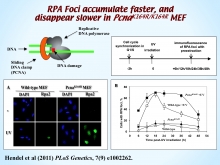
Mono-ubiquitination of PCNA is a key reaction in TLS, which enables recruitment of the specialized DNA polymerases to the damaged site in DNA through their ubiquitin-binding domain. In the yeast S. cerevisiae this reaction is essential for TLS. In mammals, however, there was a debate on whether ubiquitinated PCNA (PCNA-Ub) is essential for TLS. We probed this issue using mutant mouse cells carrying the PcnaK164R mutation, which renders them resistant to PCNA ubiquitination (in collaboration with Heinz Jacobs, NKI, Amsterdam). Direct functional analysis of TLS in these cells showed that TLS is strongly reduced across UV lesions and the cisplatin-induced GG adduct. A similar effect was obtained in cells lacking Rad18, the E3 ubiquitin ligase which mono-ubiquitinates PCNA. Consistently, cells lacking Usp1, the enzyme that de-ubiquitinates PCNA exhibited increased TLS across a UV lesion and the cisplatin adduct. In contrast, cells lacking the Rad5-homologs Shprh and Hltf, which polyubiquitinate PCNA, exhibited normal TLS. Consistently, UV-induced RPA foci, indicative of single-stranded DNA regions caused by UV, accumulated faster and disappeared more slowly in mutant mouse cells carrying the PcnaK164R mutation, compared to Pcna+/+ cells, consistent with a TLS defect. Knocking-down the expression of the TLS genes Rev3L, PolH or Rev1 in PcnaK164R/K164R MEF caused each an increased sensitivity to UV radiation, indicating the existence of TLS pathways that are independent of PCNA-Ub. Taken together these results indicate that PCNA-Ub is required for optimal TLS. However, PCNA-Ub-independent pathways exist, but operate at a significantly lower efficiency and altered mutagenic specificity.