Together with Sarah Fuchs and Ephraim Katzir, we have determined the crystal structure of a complex of α-bungarotoxin with a high affinity 13-residue peptide homologous to the binding region of the α-subunit of the AChR.
The peptide fits snugly to the toxin and adopts a β hairpin conformation. The structures of the bound peptide and the homologous loop of ACh-binding protein, a soluble analogue of the extracellular domain of AChR, are remarkably similar. Their superposition suggests that the toxin wraps around the receptor binding-site loop and, in addition, binds tightly at the interface of two of the receptor subunits, where it inserts a finger into the ligand-binding site, thereby blocking access to the ACh-binding site, thus explaining its strong antagonistic activity .
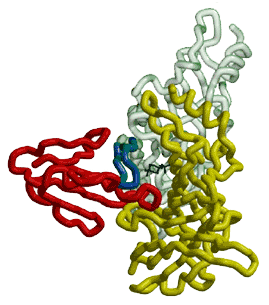
The combined model of a-BTX-HAP (red) and AChBP structure with subunit A in green and subunit B in yellow, showing the insertion of loop 2 of the toxin into the interface of the two subunits. The positively charged HEPES molecule (black stick figure) shows the location of the acetylcholine-binding site and the blockage of passage to this site caused by the binding of the toxin. The HAP, which overlaps the 182-193 loop of AChBP, is shown in blue.


