The epidermal growth factor receptor (EGFR) is involved in various cellular processes, including proliferation and motility, and its constitutive activation contributes to the transition from the primary tumor site into an invasive state leading to metastases. The ability of EGFR-family receptors to spearhead a chemotactic response relevant to tumor metastasis is actively studied in our laboratory (see the picture below). Because the initiation of motility requires MAPK activation, as well as synthesis of a new set of RNA molecules, we have concentrated on specific groups of transcripts7. Since metastases are the primary cause of cancer patients’ death, the identification of events that culminate in a robust transition to a motile cellular state is of utmost importance for the understanding of the EGF-induced metastatic process.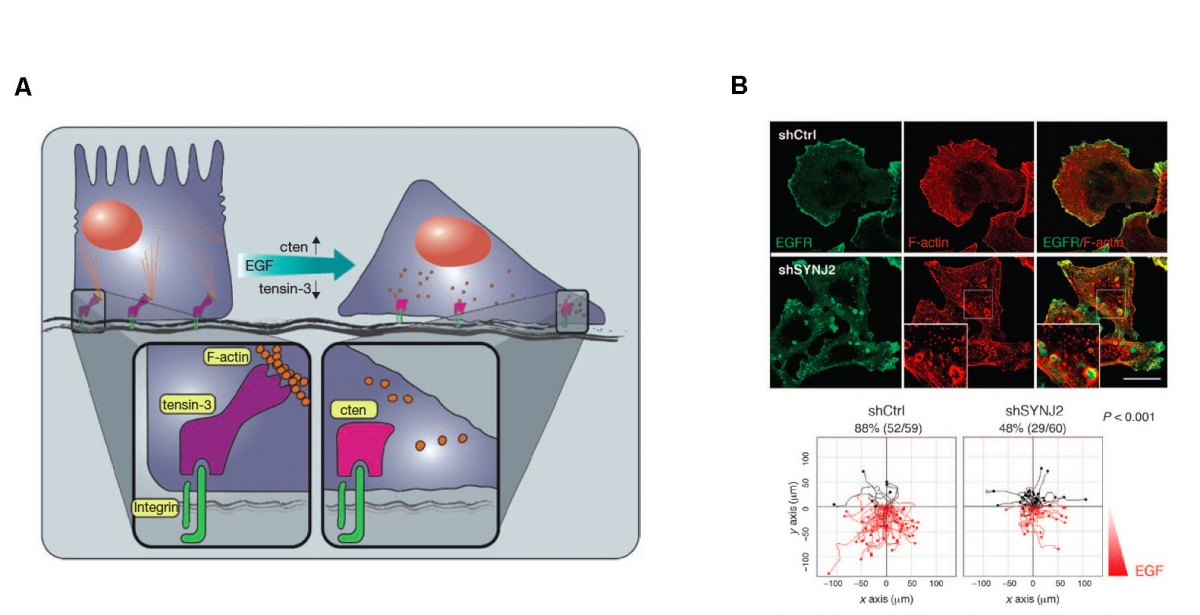
Chemotactic responses induced by EGF regulated proteins A - A model representing the functions of cten and tensin-3 in promoting EGF-induced cell migration. Resting cells, which are packed in an epithelial layer, contain stress fibers anchored to focal adhesion sites through tensin-3, or other actin binders simultaneously binding actin filaments and integrin (through a PTB domain). Following EGF stimulation, tensin-3 expression is downregulated and cten is upregulated, to a level that is sufficient for displacement of tensin-3 from integrin. Consequently, tensin-3 dissociates from focal adhesion sites, leading to the breakdown of actin stress fibres and initiation of cell migration. B - SYNJ2 localizes to lamellipodia and invadopodia and regulates endocytosis of EGFRs. Rose plots of migratory tracks of a breast cancer cell line (MDA-MB-231) cells after exposure to an EGF gradient. Red tracks mark migration toward greater EGF.


