Antibody effector functions
The response of IgG antibodies is elicited by two functional domains. Whereas the variable anti- gen-binding fragment (Fab) domain confers their antigen-binding specificity, the Fc constant domain determines antibody effector function. Effector function is achieved by engagement of this domain with Fcγ receptor (FcγR) pathways to activate innate and adaptive immune responses, including cross-presentation of antigens for the activation of T cells, antibody-dependent cell-mediated phagocytosis (ADCP), antibody-dependent cellular cytotoxicity (ADCC), and complement-dependent cytotoxicity (CDC). Research in our lab aims at understanding the factors that modulate (limit or enhance) the effector functions of both naturally occurring and therapeutic antibodies, and at identifying the cellular and molecular components that underlie successful antibody-mediated immune responses.
The role of FcγR pathways in checkpoint mAb efficacy: Checkpoint receptors in the immune synapse play a central role in regulating the immune response against tumors and are emerging as powerful targets in cancer immunotherapy. Enhancement of the patient’s own immune response against the tumor is achieved by using therapeutic antibodies to either block inhibitory checkpoints that limit the anti-tumor immune response, or to activate stimulatory checkpoints that enhance the anti-tumor immune response. Our recent studies identified unexpected FcγR-dependent mechanisms induced by several checkpoint antibodies to mediate their optimal anti-tumor activity. These studies highlighted the importance of the Fc domain of these antibodies in divergent immune responses, and identified their optimal Fc scaffold. We are studying the role of FcγR pathways in the tumor microenvironment during immunotherapy, and are characterizing interactions with checkpoint inhibitors and additional classes of therapeutic antibodies. For maximum clinical relevance, we are studying human antibodies in humanized mouse models. We are exploring strategies to enhance the identified FcγR pathways, and developing 2nd generation antibodies with improved activity.
Related recent publication: We investigated how engagement of the human FcγR by anti–PD-L1 contributes to antitumor immunity and demonstrated that beneficial FcγR signaling pathways are not engaged by existing FDA-approved mAbs. We suggested two approaches to improve their therapeutic outcome by increasing the mAb’s binding ratio of activating to inhibitory FcγR pathway activation: 1. Combining these modifications with blockade of the inhibitory receptor FcγRIIB; 2. afucosylation of the IgG1 Fc region improved antitumor responses by enabling depletion of immunosuppressive PD-L1+ cells in the tumor microenvironment alongside the blockade of inhibitory signaling in PD-1+ T cells. These findings suggest that an afucosylated IgG1 scaffold renders anti–PD-L1 mAbs more effective.
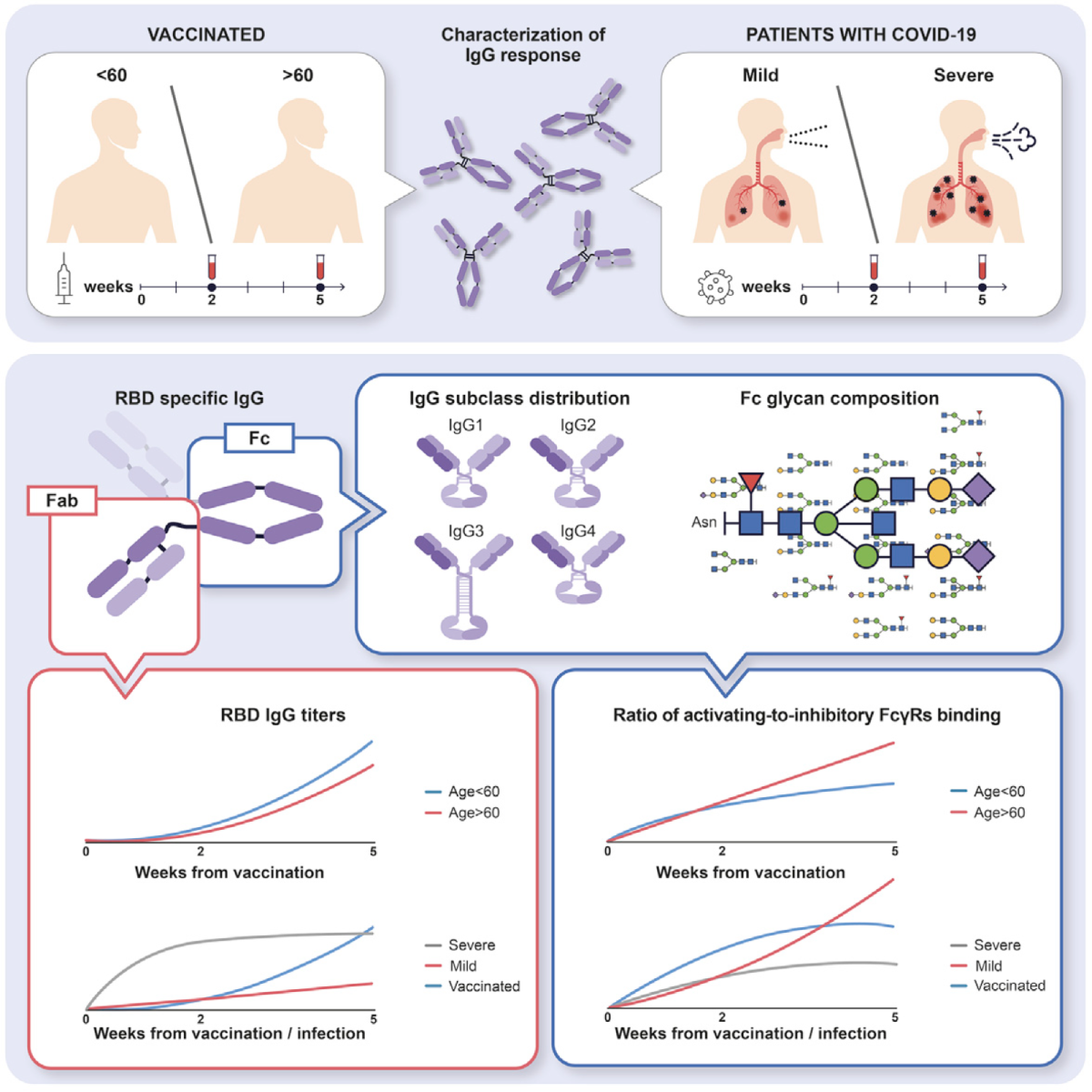
Role of IgG Fc glycome antibody response: The Fc structures of IgGs (combination of specific IgG subclasses and glycoforms) produced during infection and vaccination have important roles in shaping the immune response. The Fc glycan composition is known to dictate IgG effector functions through modulation of the IgG interactions with FcγRs and complement components. By characterizing and quantifying the IgG Fc-glycome repertoire (dozens of possible subclass-glycoform combinations) in serum samples, we aim to elucidate unique Fc-glycan profiles associated with different physiological and disease conditions, and to better understand how IgG Fc glycan structures orchestrate diverse immune responses.
Related recent publication: Anti-SARS-CoV-2 antibodies elicited by the COVID-19 mRNA vaccine exhibit a unique glycosylation pattern. We showed that vaccine- and infection-induced anti-SARS-CoV-2 IgGs differ in their Fc regions and in the engagement of complement and Fc receptors, suggesting distinct effector functions and immunity.