The DAP-Kinase Family: a Perspective
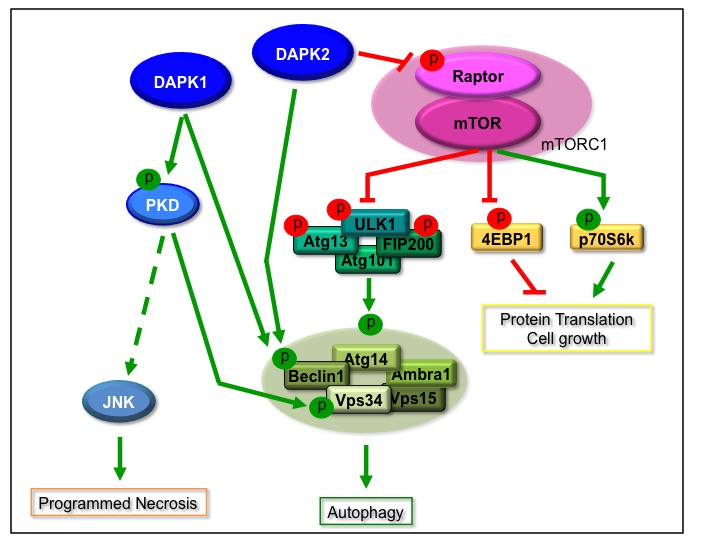
Our point-of-entry to the field of PCD came in the early 1990’s, when our lab identified the Death Associated Proteins, or DAPs, in a functional genetic screen for novel genes necessary for interferon-g induced cell death. The first to be studied was DAP-Kinase, DAPK1, a tumor suppressor with multiple functions in cell death and beyond. DAPK1 is the founding member of a group of closely related death associated Ser/Thr kinases, including DAPK2 (DRP-1) and DAPK3 (ZIPK). They all share significant homology within their kinase domains, but differ in their extra-catalytic C-terminus. In addition, we identified an isoform of DAPK2 (DAPK2b) that shares structural and functional features of both DAPK2 and DAPK3. Over a decade’s effort resulted in elucidating the function and regulation of the DAP kinases within the PCD network, mostly focusing on their role in autophagy.

Some of our ground-breaking past accomplishments include:
Identifying cell death and autophagy-relevant substrates:
- Beclin 1 (DAP1 and DAPK2)
- PKD (DAPK1)
- VPS34 (DAPK1)
- Raptor, a component of mTORC1 (DAPK2)
Context-dependent mechanisms of regulation
- binding of 14-3-3 to DAPK2’s phosphorylated tail domain
- phosphorylation of DAPK2 by AMPK during metabolic stress
- regulation by DAPK1’s GTP-binding ROC domain
Contribution to disease
- Epigenetic silencing of DAPK1 in cancer due to promoter methylation
- Recognition of tumor suppressor status of DAPK1
- Identification of DAPK3 as a critical intervention target for preventing drug resistance and potential disease relapse in melanoma following classical treatment with BRAF inhibitors