All organisms produce a large repertoire of gene-encoded cationic antimicrobial peptides (AMPs), which serve to protect the host from microbial invasion. Most of these molecules directly kill bacteria by targeting and perforating the negatively charged membrane of the pathogen. In light of a worldwide emergence of drug-resistance bacterial pathogens, AMPs are promising therapeutic candidates.
Another property of AMPs is that they can bind and neutralize bacterial lipopolysaccharides which are potent activators of Toll-like receptors in different innate-immune cells. Their release to the blood is the primary cause of septic shock.
We combine biological and biophysical methods to understand:
- The mechanism of action of AMPs against planktonic and biofilm-embedded bacterial pathogens.
- How bacteria develop resistance to AMPs
- The mechanism of lipopolysaccharides neutralization by AMPs.
The Carpet Model
- Since 1991 we have established the “carpet” mechanism as a model describing an efficient membrane permeation process used by many antimicrobial peptides, as compared to the “barrel-stave” mechanism used by peptide ion channels. Most importantly, we disproved accepted dogmas on the role of a specific structure, sequence or chirality in biological function. Furthermore, we showed that peptide assembly in solution and particularly in membranes is a crucial parameter controlling target cell specificity.
- The success of our model is also reflected by our ability to develop a novel family of cell selective antimicrobial diastereomeric peptides based on predictions not possible by other models. This new family seems to have the highest potential for future therapeutics, urgently needed due to increasing resistance of bacteria to available antibiotics. Indeed, they presented the first example of antimicrobial peptides that were active against bacterial infection when injected intravenously.

Figure 1: The barrel-stave and the carpet models suggested for membrane permeation. In the carpet model the peptides interact only with the lipid head groups, whereas in the barrel-stave model peptides insert into the lipid core. The destruction of a cancer cell is shown as an example.
Anticancer Peptides
Based on existing evidence that antimicrobial peptides can also kill cancer cells in vitro, we moved forward and de-novodesigned a diastereomeric peptide with high selectivity toward cancer cells and determined its mode of action. Most importantly, the peptide was highly potent in animal models of primary and metastatic tumors when injected intratumor or intravenously (the first reported study). In contrast, the same peptide, but composed of all L-amino acids, was highly active only in vitro and could not discriminate between tumor and non-tumor cells.
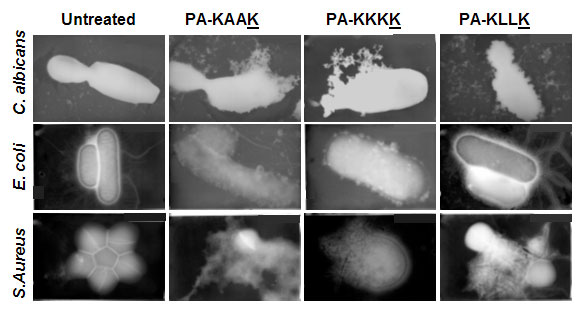
Figure 2: Lysis of bacteria and fungi induced by ultrashort lipopeptides. PA designates palmitic acid; K designates Lysine; A designates Alanin; and L designates Leu. Underlined amino acid is the D-optical isomer.