Disulfide Bonding in Mucin Assembly
Using cryo-electron microscopy, X-ray crystallography, and biochemistry, we have shed light on the mechanisms by which mucins form disulfide-linked polymers.
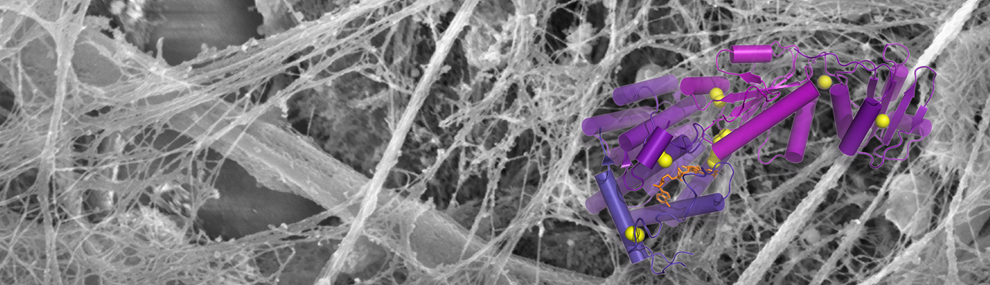
Using cryo-electron microscopy, X-ray crystallography, and biochemistry, we have shed light on the mechanisms by which mucins form disulfide-linked polymers.
The QSOX1 enzyme regulates glycan modification in the Golgi by introducing activating disulfides into glycosyltransferases.
We have developed a monoclonal antibody that binds and inhibits human QSOX1 with an inhibition constant of about 1 nM.