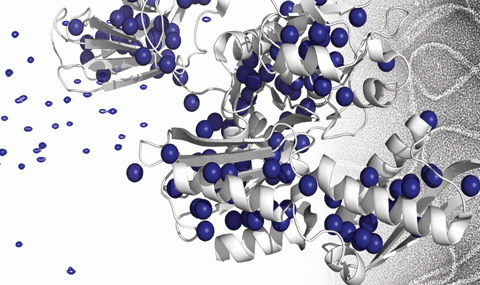
Conformational changes in and within the disaggregation machinery chaperones
Cells employ sophisticated quality control systems to ensure that cellular proteins perform their intended functions. Under stress conditions, however, proteins folding can start to go wrong, causing the accumulation of misfolded proteins and formation of toxic protein aggregates. Excessive accumulation of such misfolded proteins and/or aggregates can overwhelm the cell's quality control machinery, leading to loss of function and, consequently, cell death. Hsp104/ClpB molecular chaperones, however, can help reverse these toxic effects by forcibly untangling protein aggregates and allowing the client proteins to refold into their native states.
Our lab uses NMR spectroscopy to study the conformational changes and allosteric interactions within and between different domains of Hsp104/ClpB in order to understand how these chaperones regulate their activity.
Moreover, we aim to explore how regulatory factors, binding partners, and substrates influence the activity of this machinery, and how these dynamics processes affect the protein disaggregation function.
NMR spectroscopy is the method of choice for studying these types of transient and dynamic interactions, as we can probe a wide range of protein motions (from the nanosecond time-scale up to the second time-scale), in a near cellular environment and with atomic resolution. The state-of-the-art NMR techniques used in our lab further enable us to study very large molecular systems (> 1.5 MDa) such as the hexameric Hsp104/ClpB chaperones. Using these ultra-sensitive observations of methyl-groups in the proteins, in combination with segmental labelling, then allows us to monitor the structure, dynamics and interactions of the disaggregation system.