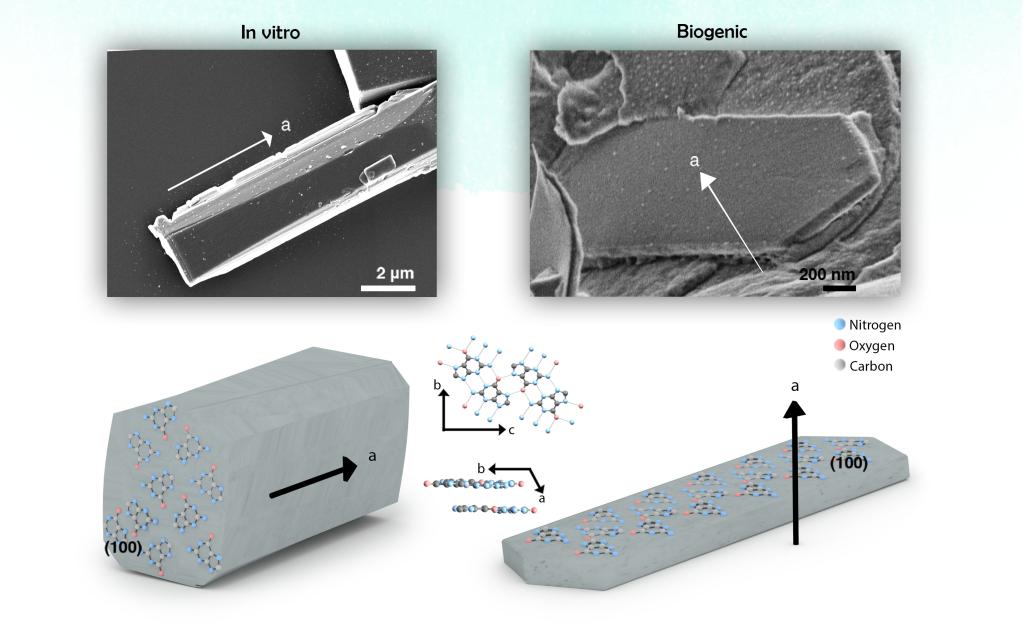
Controlling the morphology of crystalline materials is challenging, as crystals have a strong tendency toward thermodynamically stable structures. Yet, organisms form crystals with distinct morphologies, such as the plate-like guanine crystals. Guanine crystals are constructed from H-bonded molecular layers that are stacked one on top of the other by π-stacking, causing an inherent thermodynamic tendency for the crystals to grow low-energy prismatic structures (Figure 1, in vitro). Many organisms, however, produce plate-like guanine crystals that expose the extremely high, in-plane refractive index (n = 1.83) to light, thereby allowing the construction of highly effective and versatile photonic arrays (Figure 1B, biogenic).
In order to elucidate the mechanism that allow such tight and extensive control over crystal morphology the Gur’s lab, in collaboration with the EM team, studied the zebrafish model organism, under cryogenic conditions, using variety of high resolution imaging approaches. For capturing the initial transient stages of crystal formation in the context of the overall tissue, cryo scanning electron microscopy in 2D (freeze fracture) and 3D (cryo FIB/SEM) was performed on high pressure frozen tissues. For nanometric scale insights, cryo-electron tomography (cryoET) was utilized on plunged frozen iridophores that were isolated from the organism.

To correlate morphological information with crystallographic features, cryogenic 4D scanning transmission electron microscopy (cryo 4D-STEM) was used to collect an electron diffraction pattern from every point the beam raster traverses.
The overall information reveals that guanine crystals form via templated nucleation of thin leaflets on preassembled scaffolds made of 20-nm-thick amyloid fibers. These leaflets then merge and coalesce into a single plate-like crystal. These findings shed light on the biological regulation of crystal morphogenesis, which determines their optical properties.

Ref: Eyal Zohar, Rachael Deis, Neta Varsano, Nili Dezorella, Katya Rechav, Lothar Houben, and Dvir Gur. J. Am. Chem. Soc. 2022, 144, 49, 22440–22445

