Enabling and understanding new methodologies to fabricate molecular assemblies driven by
intermolecular interactions is fundamental in chemistry. Such forces can be used to control crystal growth and enable surface-confinement of these materials, which remains challenging. In the 1960s,
the late Gerhard M. J. Schmidt from the Weizmann Institute of Science discovered that some
molecules are unusually closely arranged in organic crystals. This spatial arrangement turned out to
be a result of an unexplored weak intermolecular interaction, termed “halogen bonding” many years
later by other scientists. Such interactions play a key role in organic processes (in a cell or organism)
that are necessary for sustaining life. For example, thyroid hormones use halogen bonding to bind to
receptors in order to regulate the development, growth and metabolism of the cells in our body. We
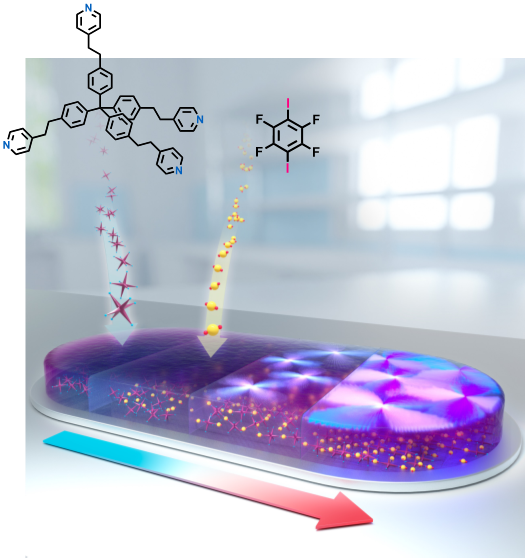
recently demonstrated a solvent-free on-surface crystal-to-co-crystal conversion process driven by
halogen bonding. By exposing a polycrystalline organic material, consisting of a halogen
bonding–acceptor moiety, to the vapors of a complementary halogen bonding–donor compound, the
corresponding co-crystals were formed. Furthermore, we show that this approach can also be utilized
for non-crystalline materials to afford surface-confined organic composites. This crystallization
process can be followed by optical microscopy as shown in this video. Our stepwise vapor-based
approach offers a new strategy for the formation of hybrid supramolecular materials.
- Non-Covalent Bonding Caught in Action: From Amorphous-to-Cocrystalline Molecular Thin Films
Chovnik, O.; Cohen, S.; Pinkas, I.; Houben, L.; Gorelik,T.; Feldman, Y.; Shimon, L.; Iron, M.; Lahav, M.; van der Boom, M. E.
ACS Nano 2021, 9, 14643–14652. - Directed Molecular Structure Variations of Three-Dimensional Halogen-Bonded Organic Frameworks (XBOFs)
Shankar, S.; Chovnik, O.; Shimon, L.; Lahav, M.; van der Boom, M. E.
Cryst. Growth Des., 2018, 18 , 1967-1977. - Finding the Perfect Match: Halogen vs. Hydrogen Bonding Shirman, T.;
Boterashvili, M.; Orbach, M.; Freeman, D.; Shimon, L. J. W.; Lahav, M.; van der Boom, M. E.
Cryst. Growth Des., 2015, 15, 4756-4759.


