Breaking their teeth on an immune disorder
A newly discovered autoimmune disorder disrupts tooth enamel development, with implications for the earlier diagnosis of celiac disease
Briefs

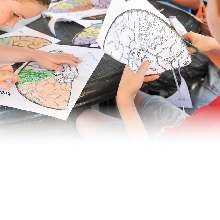
Scanning electron microscope images of enamel structure that developed abnormally after attacks by autoantibodies against enamel proteins. (Image from the Abramson lab)
A recent study by Prof. Jakub Abramson and his team in the Department of Immunology and Regenerative Biology has revealed that the mechanism by which a rare children’s autoimmune disorder, which hinders proper tooth enamel development, may enable earlier detection and prevention of celiac disease.
Enamel, the hardest and most mineral-rich substance in the human body, covers and protects our teeth. But in one-third of children with celiac disease, this layer is defective, failing to protect the teeth properly. As a result, teeth become more sensitive to heat, cold, and sour food, and they may decay faster.
People with a rare genetic disorder known as APS-1 (autoimmune polyglandular syndrome type 1) suffer from a variety of autoimmune diseases in addition to the faulty development of enamel on their permanent teeth. Prof. Abramson hypothesized that this defect may also be of an autoimmune nature.
The Abramson lab investigates the establishment of immunological tolerance, a process in which the immune system (e.g., T cells) “learns” to recognize and tolerate the body’s own components. In the thymus gland, nascent T cells are effectively given a survey course of virtually all proteins that are expressed in the body. Learning in the thymus about how the body proteins look like is essential for preventing the immune system from mounting a “friendly fire” attack on body’s own tissues that could eventually result in autoimmune disorders. In APS-1 patients, a mutation in a gene known as the autoimmune regulator (AIRE) leads to ineffective T cell education.
In their study, published last year in Nature, the Abramson lab examined how mutations in AIRE could lead to defective enamel. The researchers discovered that in the absence of the protein the AIRE gene normally encodes, enamel (and other) proteins are excluded from the survey course. As a result, “functionally illiterate” T cells graduate from immune school and encourage the production of antibodies to the enamel proteins that are critical for the formation of permanent teeth. The reason baby teeth are unaffected is that the immune system isn’t fully operational and capable of producing anti-enamel antibodies until at least age six.
But why do some people with celiac disease suffer from imperfect enamel? In celiac disease, gluten triggers T cells to attack and destroy the cells lining the small intestine— despite the fact that these misguided T cells received an otherwise “proper” education.
Collaborating with clinicians, Prof. Abramson and his team analyzed biospecimens from celiac patients who had undergone an endoscopy procedure. They found that a substantial number of celiac patients had the same anti-enamel antibodies as APS-1 patients. The researchers hypothesized that those proteins present in both the intestine and the dental tissue could play an important role in enamel development. In this case, the antibodies that identify proteins in the intestine might move through the bloodstream to the dental tissue, where they could disrupt the enamel production process.
Moreover, many celiac patients develop sensitivity to cow’s milk. The Abramson group, therefore, focused on the kappa (k)-casein protein, a major component of dairy products. Strikingly, they found that the human equivalent of k-casein is one of the main components necessary for enamel formation. The researchers hypothesized that antibodies produced in the intestines of celiac patients against k-casein may subsequently cause collateral damage to the development of enamel in the teeth, similar to the way in which antibodies against gluten can trigger autoimmunity against the intestine.
These findings could have implications for the food industry. “Similar to gluten, the consumption of large quantities of dairy products could lead to the production of antibodies against k-casein,” Prof. Abramson explains. “This protein increases the amount of cheese that can be produced from milk, so the dairy industry deliberately raises its concentration in cow’s milk. Our study, however, found that k-casein is a potent immunogen, which may potentially trigger an immune response that can harm the body itself. Most important, early diagnosis in children may enable preventive treatment in the future.”
JAKUB ABRAMSON IS SUPPORTED BY:
-
Dr. Joseph Bollag and Sarah Sharabi-Bollag
-
Bill and Marika Glied and Family Fund
-
Israel Makov
-
Moross Integrated Cancer Center
-
Sy Syms Foundation
-
Eugene and Marcia Applebaum Professorial Chair