Although it is well established that tumor-specific CTLs enter primary tumors (e.g., raised against patient specific neoantigens or tumor-enriched antigens), the ability to use similar tumor-specific CTLs to eradicate secondary tumors (metastasis) at remote organs has been very limited because these injected CTLs must be able to exit (extravasate) blood vessels nearby the metastatic lesions they are assigned to eliminate. The lung is a major organ of metastasis of multiple malignancies including melanoma, breast cancer and lung cancer. Currently, the feasibility of targeting tumor specific killing T cells through the dense vasculature of the lung to enter metastatic lesions and kill these lesions is unclear. Using syngeneic immune-competent models of lung metastasis we dissect if and how lung vessels nearby metastatic lesions undergo specific changes (e.g., upregulation of specific vascular cell adhesion molecules and chemokines, remodeling of endothelial junctions) and how factors released both the primary tumor and by the micro-metastatic lesions account for these changes. We also dissect how effector CTLs with defined antigen specificity translate these molecular changes into exit signals that allow these T cells to leave the blood vessels nearby metastatic lesions and kill these lesions before they expand into macroscopic tumors. We plan to test if the TCRs of tumor specific CTLs is used not only for killing tumors and metastatic lesions but also for recognizing antigenic signals which might serve as effective extravasation and infiltration signals for the tumor specific CTLs (TILs). The ability to follow the migration and communication of specific cytotoxic T cells with specific cells within metastatic lesions by real time microscopy is limited by the very small volume of tissue imaged by available imaging methods. We therefore introduced LSM as a state of the art tool to scan large volumes of lipid cleared lung lobes in search for specific locations of tumor specific CTLs entering metastatic lesions that express specific antigenic peptides recognized by these CTLs. Newly developed in situ intravascular and intra-airway immunostaining techniques combined with tagging metastatic cancer cells of interest allows us to dissect the molecular basis of metastatic lesion eradication by killer CTLs (TILs) in various compartments of the lungs.

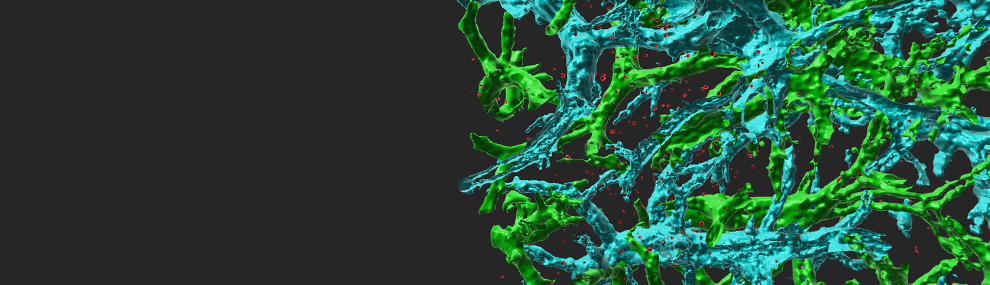
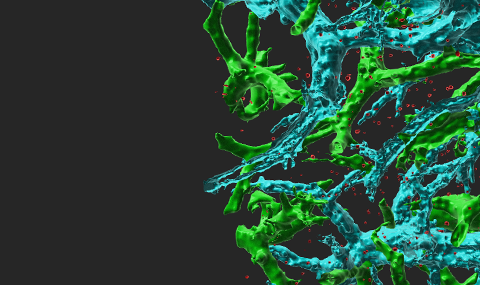
Fluorescent labeled breast cancer cells growing inside the lungs of recipient immunocompetent mice The lung vessels are stained with cyan labeled anti-CD31. Note the high fraction of cancer cells (red) residing inside the lung alveoli.