Rational design of endogenous-like inhibitors and modulators targeting specific matrix remodeling enzymes
Controlling the enzymatic activity of specific ECM remodeling enzymes by antagonist molecules is highly desirable, first, for studying their individual function and mode of action, and second as potential therapeutic agents. However, the design of antagonists targeting a specific enzyme often appears to be an extremely difficult task. The development of such inhibitors has evolved over the years and great progress was achieved in the last decade in understanding the biochemistry and biology of the enzymes' activity. This knowledge, combined with lessons from the past has drawn new approaches for the development of next-generation enzyme inhibitors..
Our group deals with the design of potent and selective inhibitors for human matrix metalloproteinases, ADAMs, LOXs and LOXLs. Our design is based on detailed mechanistic information obtained from our molecular and biophysical studies. We solve the molecular architecture of the natural inhibitors of matrix proteases, TIMPs and enzyme pro-domains by protein X-ray crystallography/NMR techniques and study their mechanism of inhibition in our biophysics, biochemistry and cell biology set ups. In addition, we use protein engineering and directed evolution methodologies to re-design and stabilize recombinant protein-based modulators targeting key enzymes that remodel the cellular microenvironment. We utilize the Yeast Surface Display (YSD) system to express and facilitate protein engineering, creating a novel high-throughput screening system for the directed evolution of ECM modifying enzymes. Nowadays, we also take inhibitor evolution a step further by developing molecular agents with improved binding specificity towards selected physiological substrates, producing a set of pathway-specific inhibitors.
Our group developed and characterized function-blocking antibodies, which bind and inhibit specific ECM enzymes and display therapeutic potential (Sela-Passwell et al., Nature Medicine 2012), (Grossman et al., Cancer Research 2016), (Udi et al., Structure 2015), (Talmi-Frank et al., Cell Host & Microbe 2016). In addition, we have produced and stabilized a recombinant pro-domain of ADAM17, which blocks TNFα shedding and also displays therapeutic potential in various disease models (Wong et al., Scientific Reports.2016), (Wong et al., The Journal of Biological Chemistry 2015).
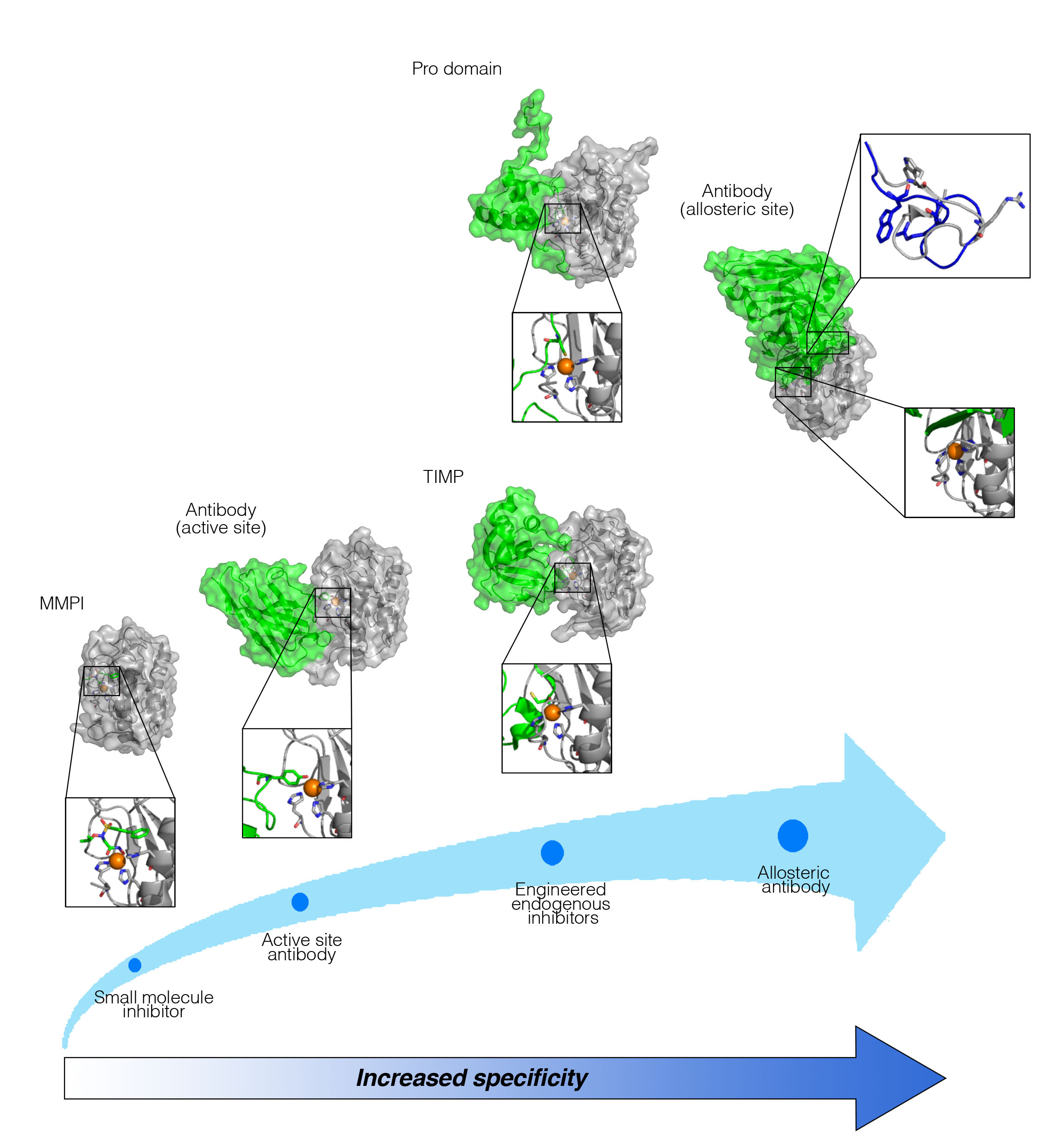
"Evolution" of MMP inhibitors. (Levin et al., Biochimica et Biophysica Acta - Molecular Cell Research, 2017)


















