The role of DC ICAM-1 in immune synapses in vivo: from vaccination to cancer immunity
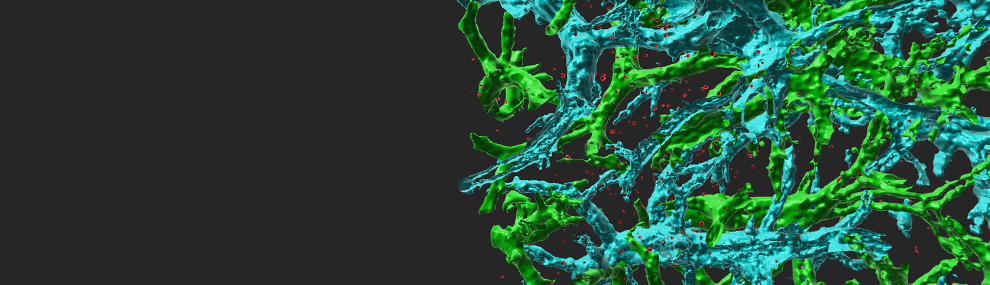
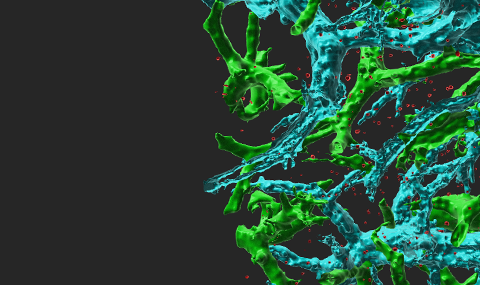
ICAM-1 is major cell adhesive ligand of the leukocyte integrin LFA-1. It is expressed on various vascular cells, pericytes, on different leukocytes including antigen presenting dendritic cells (DCs), macrophage subsets and B cells, as well as on various transformed epithelial cells. We have recently found that functional contacts between CD4 lymphocytes and adjuvant stimulated dendritic cells (DCs) inside lymph nodes (a model for skin vaccination) take place independently of ICAM expression on these DCs (Scheme 1). Our work shows that priming and Th1 differentiation of these lymphocytes depend primarily on TCR signals rather than on T cell integrins (Movie 1). In another vaccination model using migratory antigen loaded DCs injected into the skin, we learned that naïve T cells can use their integrins to ICAM on these DCs but under restricted conditions, further suggesting that this ligand is dispensable for DC based vaccinations. We now extend these findings to new infection models in genetically engineered mice. We have also recently established a new influenza virus infection model in mice based on the H1N1 murine adapted PR8 strain (Movie 2). In parallel, we recently generated the first dendritic cell specific ICAM-1 knockout mice and plan to follow in them the contribution of DC ICAM-1 to priming, differentiation and memory generation of subsets of CD8 and CD4 T cells critical for influenza clearance and long lasting immunity. These studies will be extended to additional viral infection models in the skin. Collectively, our studies raise the possibility that naïve T cells can not activate and use their integrins to bind specific ICAMs on DCs. Once differentiated into effector CD4 and CD8 T cells and exiting lymph nodes, T cells acquire unique adhesive properties that allow them to bind ICAMs presented by tissue macrophages, DCs and stromal elements. Whether these programs are essential for the ability of these effector T cells to clear infectious agents in tissues is an open question we plan to investigate. Another research direction we will take is to define if effective anti-tumor immunity depends on the presence of ICAM-1 on tumor cells (e.g., melanoma, breast carcinoma) and/or on different subsets of DCs, monocytes, and macrophages in tumor draining lymph nodes and in the tumor microenvironment.

Scheme 1 Major findings of our work on the use of ICAMs by naïve CD4 T cells entering vaccinated skin draining lymph nodes Each step in the cascade of lymphocyte entry, search for antigen, activation and proliferation is depicted and the role of ICAM-1 and ICAM-2 in the respective step is indicated. Naive CD4 T cells entering Th1-vaccinated LNs arrest normally on resident LN DCs devoid of ICAMs. T cell contacts with resident LN DCs devoid of ICAMs promote Th1 and Tfh differentiation. A fraction of CD4 T cells use ICAM-1 to arrest on migratory bone marrow derived DCs injected into the skin and entering the same lymph nodes but these firm adhesions are not necessary for CD4 T cell activation and proliferation.