Cytokine-induced expansion of hematopoietic stem and progenitor cells (HSPCs) is not fully understood.
In the present study, we show that whereas steady-state hematopoiesis is normal in basic fibroblast growth factor (FGF-2)–knockout mice, parathyroid hormone stimulation and myeloablative treatments failed to induce normal HSPC proliferation and recovery.
In vivo FGF-2 treatment expanded stromal cells, including perivascular Nestin supportive stromal cells, which may facilitate HSPC expansion by increasing stem cell factor (SCF) and reducing CXCL12 via mir-31 up-regulation.
FGF-2 predominantly expanded a heterogeneous population of undifferentiated HSPCs, preserving and increasing durable short- and long-term repopulation potential. Mechanistically,these effects were mediated by c-Kit receptor activation, STAT5 phosphorylation, and reduction of reactive oxygen species levels.
Mice harboring defective c-Kit signaling exhibited abrogated HSPC expansion in response to FGF-2 treatment, which was accompanied by elevated reactive oxygen species levels. The results of the present study reveal a novel mechanism underlying FGF-2–mediated in vivo expansion of both HSPCs and their supportive stromal cells, which may be used to improve stem cell engraftment after clinical transplantation.
[figure:center:1]

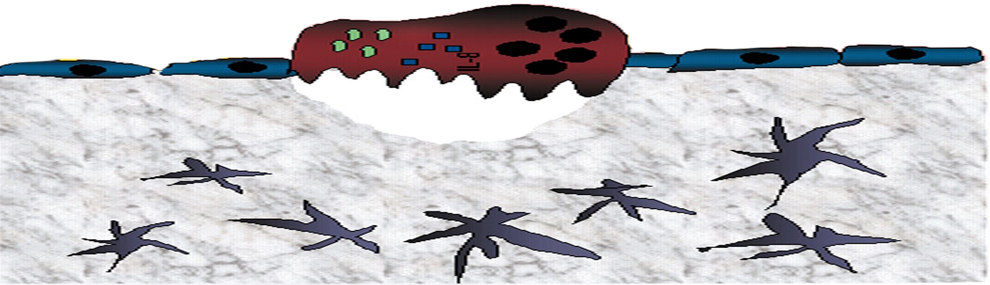
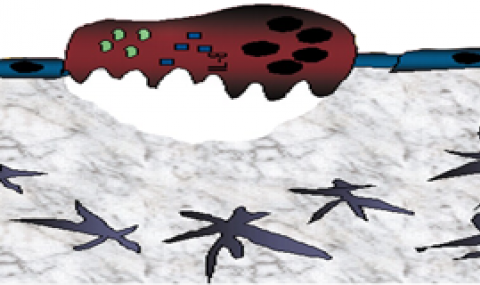
Figure 2. At steady-state conditions, HSPCs reside in proximity to “niche” supportive Nestin mesencymal stem cells (MSC). FGF-2 is expressed locally in low levels, allowing low-key steady-state maintenance of HSPCs. CXCL12 is expressed at high levels, keeping HSPCs in a quiescent state. After FGF-2 treatment, a dual increase in the Nestin MSC and the HSPC pool size occurs. Stromal CXCL12 levels are reduced via mir-31 up-regulation, allowing HSPCs to exit from their quiescent state and begin cycling. Stromal SCF levels are up-regulated, activating c-Kit, which is highly expressed by FGF-2–stimulated HSPCs. Activated c-Kit expands the pool of HSPCs and increases long-term repopulating HSC capacity by decreasing intracellular reactive oxygen species (ROS) levels and activating stem cell–maintaining factors such as STAT5.
[figure:center:1]