Our work is conducted in close collaboration with Professor Mudi Sheves and Professor Israel Pecht.
Coherent Transport through Azurin Monolayers
Electron transport across proteins (and across molecules, in general) is strongly influenced by coupling between the electrons in the electrodes and those in the molecules. We analyse the differences in I-V and conductance (dI/dV) results obtained between two different configurations: Au-Azurin-Au (fig. A) and Au-Azurin/linker-Au (fig. B) junctions at low temperatures (10K).
The I-V characteristics obtained for the Azurin junction with a monolayer of saturated (redox-inactive) linker molecules, covalently bound to one of the Au electrodes, leads to non-linear I-V behaviour in contrast to what is the case without the linker (Au-Azurin-Au), at low temperatures (~10K) the first derivative of the current (dI/dV) as a function of voltage shows a peak-like structure, rather than the step-like increase of conductance. Insertion of the short (< 1 nm) linker molecule weakens the coupling of the Azurin to one of the Au electrodes, while keeping the strong coupling to the other Au electrode (via a Au-S bond) the same in both junctions. While our data could be explained by Coulomb blockade, temperature-dependent results and theoretical modelling provide strong evidence for resonant tunneling through discrete energy levels as the cause for this behaviour.
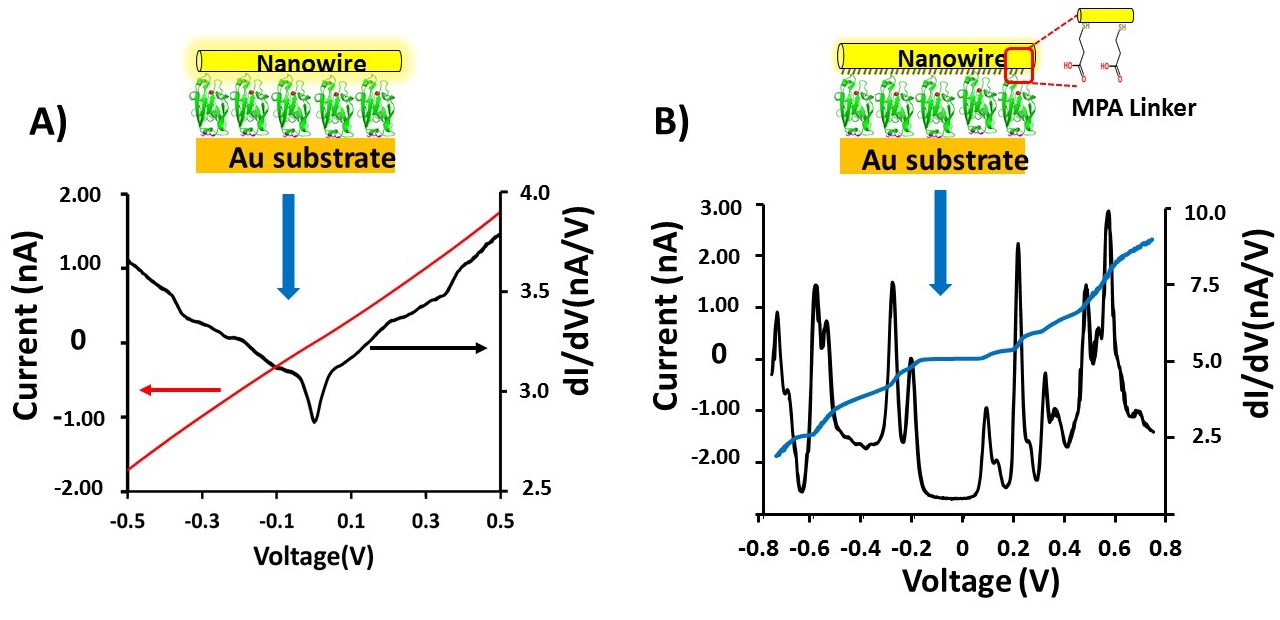
A (top left), current – voltage, I-V (red) and conductance voltage, dI/dV – V (black) plots of the Az junction between -0.5 and +0.5 V.
Transistor Configuration Yields Energy Level Control in Protein-Based Junctions
The incorporation of proteins as functional components in electronic junctions has received much interest recently due to their diverse bio-chemical and physical properties. However, information regarding the energies of the frontier orbitals involved in their electron transport has remained elusive. Here we employ a new method to quantitatively determine the energy positions of the molecular orbitals, nearest to the Fermi level of the electrode, in the electron transfer protein Azurin. The importance of the Cu(II) redox centre of Azurin is demonstrated by measuring gate-controlled conductance switching which is absent if Azurin's copper ions are removed. Comparing different electrode materials, a higher conductance and a lower gate-induced current onset is obseved for the material with smaller work function, indicating that electron transport via Azurin is LUMO-mediated. We use the difference in work function to calibrate the difference in gate-induced current onset for the two electrode materials, to a specific energy level shift and find that electron transport via Azurin is near resonance. Our results provide a basis for mapping and studying the role of energy level positions in (bio)molecular junctions.
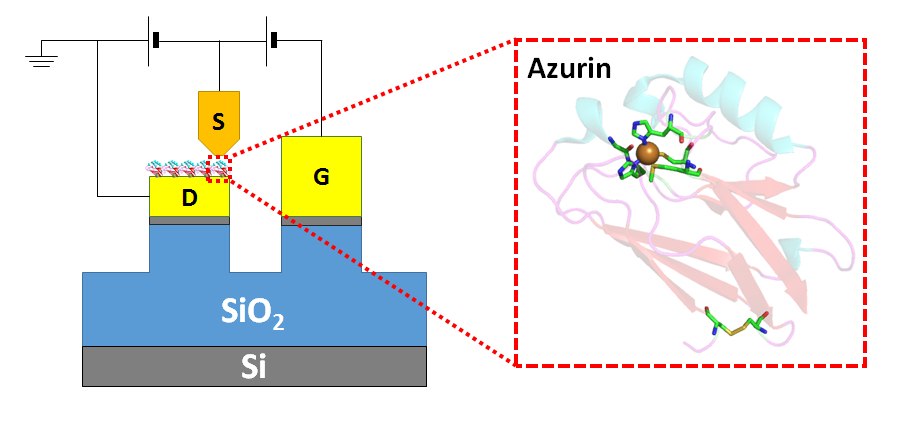
Source-drain-gate configuration in a conducting probe AFM set up. The protein Azurin is shown, immobilised on the drain.
Interface Electrostatics Dictates the Electron Transport via Bioelectronic Junctions
Different batches of Si wafers with nominally the same specifications were found to respond differently to identical chemical surface treatments aimed at regrowing Si oxide on them. We found that the oxides produced on different batches of wafer differ electrically, thereby affecting solid-state electron transport (ETp) via protein films assembled on them. These results led to the another set of experiments, where we studied this phenomenon using two distinct chemical methods to regrow oxides on the same batch of Si wafers. We have characterized the surfaces of the regrown oxides and of monolayers of linker molecules that connect proteins with the oxides and examined ETp via ultrathin layers of the protein bacteriorhodopsin, assembled on them. Our results illustrate the crucial role of (near) surface charges on the substrate in defining the ETp characteristics across the proteins. This is expressed most strikingly in the observed current’s temperature dependences, and we propose that these are governed by the electrostatic landscape at the electrode–protein interface rather than by intrinsic protein properties. This study’s major finding, relevant to protein bioelectronics, is that protein–electrode coupling in junctions is a decisive factor in ETp across them. Hence,surface electrostatics can create a barrier that dominates charge transport and controls the transport mode across the junction. Our findings’ wider importance lies in their relevance to hybrid junctions of Si with (polyelectrolyte) biomolecules, a likely direction for future bioelectronics. A remarkable corollary of presented results is that once an electron is injected into the protein, transport within the proteins is so efficient that it does not encounter a measurable barrier down to 160 K.
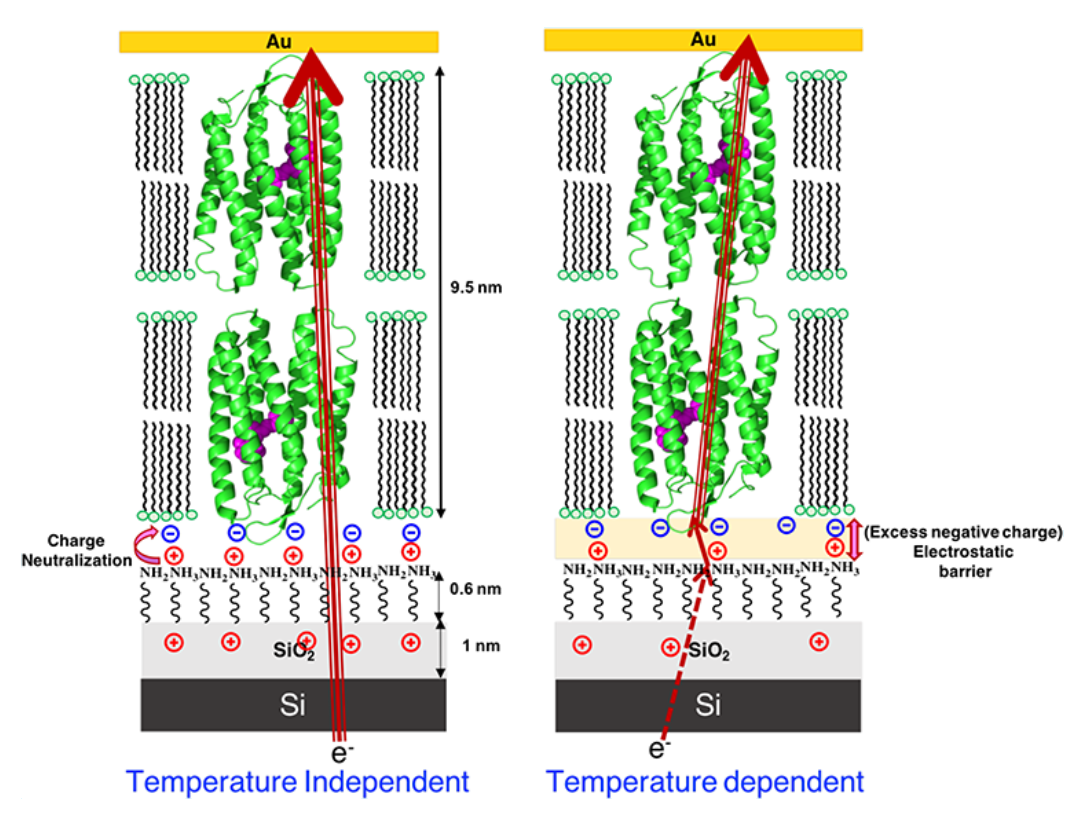
Schematic presentation of electron transport mechanism through OTG-bR on Si, showing that (left) when the positive charge on the substrate surface is higher due to complete neutralization of charge, there is good coupling between the electrode and protein, inducing near barrier-less electron transport, hence temperature independent electron transport. If the surface is less positively charged (right), due to incomplete neutralization of the proteins negative charge, an electrostatic barrier is induced and temperature dependent electron transport is observed.
Conductance in Multi-Heme Cytochromes
Multi-heme cytochrome c (Cytc) proteins are key for transferring electrons out of cells, to enable intracellular oxidation to proceed in the absence of O2. In these proteins most of the hemes are arranged in a linear array suggesting a facile path for electronic conduction. To test this, we studied solvent-free electron transport across two multi-heme Cytc-type proteins: MtrF (deca-heme Cytc) and STC (tetra-heme Cytc). Transport is measured across monolayers of these proteins in a solid state configuration between Au electrodes. Both proteins showed 1000× higher conductance than single heme, or heme-free proteins, but similar conductance to monolayers of conjugated organics. Conductance is found to be temperature-independent (320–80 K), suggesting tunneling as the transport mechanism. This mechanism is consistent with I–V curves modelling, results of which could be interpreted by having protein-electrode coupling as rate limiting, rather than transport within the proteins.

Inside front cover in Chemical Science, Volume 9, Number 37, 7 October 2018 (link)
Electron Transport via Proteins
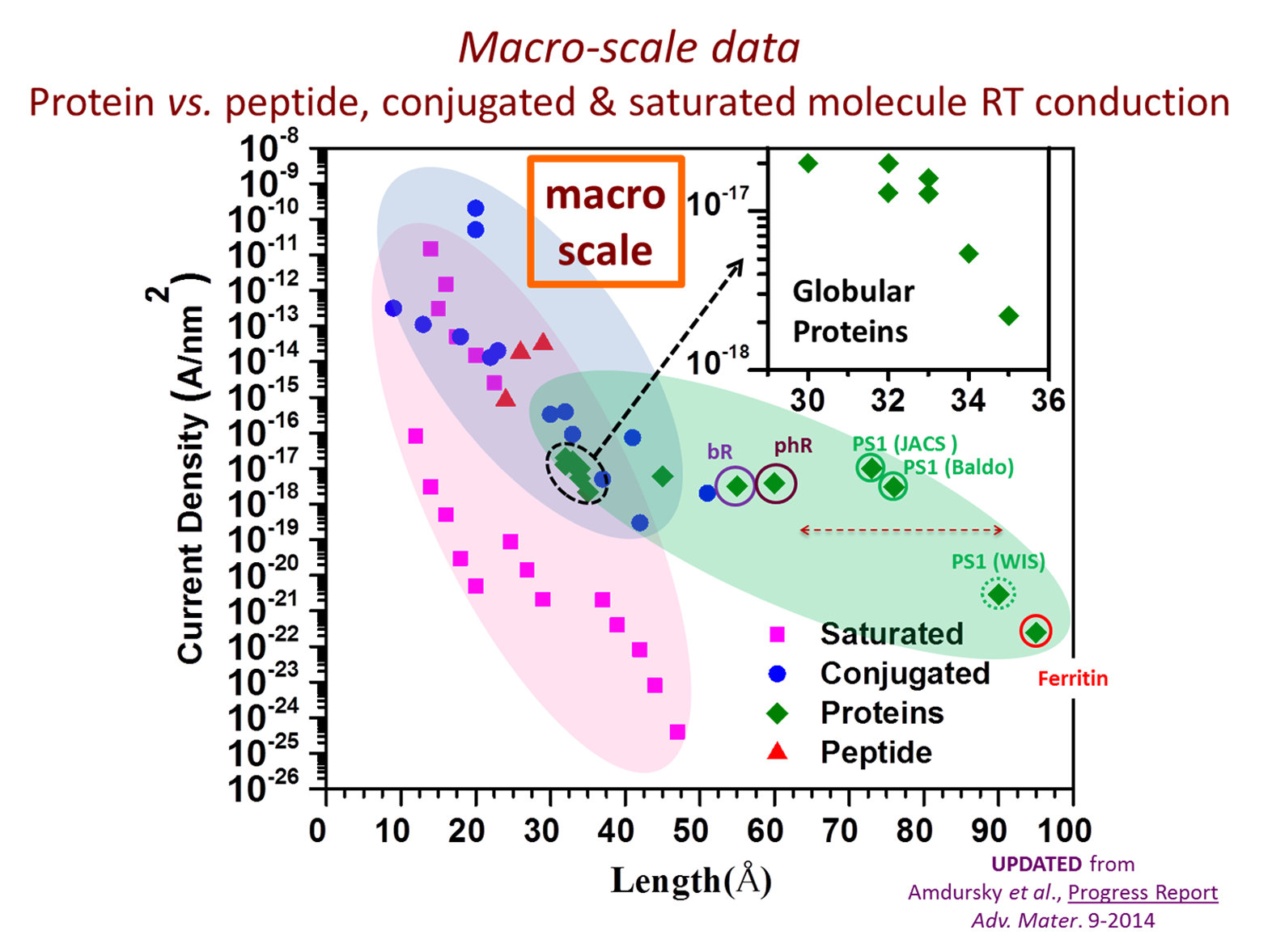
A central vision in molecular electronics is the creation of devices with functional molecular components that may provide unique properties. Proteins are attractive candidates for this purpose, as they have specific physical (optical, electrical) and chemical (selective binding, self-assembly) functions and offer a myriad of possibilities for (bio-)chemical modified cation. Our group focuses on proteins as potential building components for future bio-electronic devices as they are quite efficient electronic conductors, compared with saturated organic molecules. We several questions: how general is this behavior; how does protein conduction compare with that of saturated and conjugated molecules; and what mechanisms enable efficient conduction across these large molecules? To answer these questions results of nanometer-scale and macroscopic electronic transport measurements across a range of organic molecules and proteins are compiled and analyzed, from single/few molecules to large molecular ensembles and the influence of measurement methods on the results is considered. Generalizing, it is found that proteins conduct better than saturated molecules, and somewhat poorer than conjugated molecules. Significantly, the presence of cofactors (redox-active or conjugated) in the protein enhances their conduction, but without an obvious advantage for natural electron transfer proteins. Most likely, the conduction mechanisms are hopping (at higher temperatures) and tunneling (below ca. 150–200 K).
Electrostatic Gating of Solid-State Proteins in E-beam Defined Nano-gaps
Temperature and length dependence are common tools for determining electron transport mechanisms in (bio)molecular electronics and is often used to distinguish a transition between tunneling and hopping transport. Here we employ similar methods to study transport in proteins. After immobilizing proteins in a planar source-drain nano-junction, a third electrode can be used to electrostatically gate the bound protein. This applied gate field can shift the electrostatic potential of the protein. By further applying a source-drain bias the Fermi levels of the electrodes can be manipulated, aligning them with the electrochemical potential of the protein. This measurement could provide insight into the energy level structure of the protein.
Furthermore, by measuring both temperature and gate dependence, the activation energy of transport can be controlled by bringing the protein into and out of (near) resonance with the electrodes. This control over the injection barrier (and/or multi-step hopping barrier) could mean a control over the conduction mechanism in a junction; from temperature-independent coherent tunneling to incoherent temperature-dependent sequential hopping transport – or something in-between.
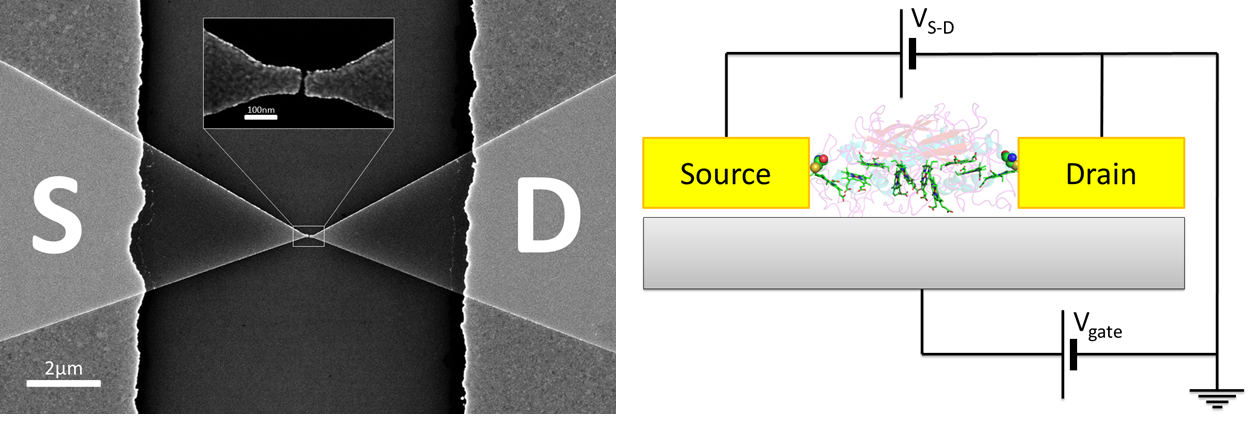
SEM image of e-beam defined nanogaps (left) and a schematic of a gated protein in solid state (right)
Inelastic Tunneling Spectroscopy (IETS)
Exploring the interaction between transported electrons and molecular vibrations (phonons) is at the forefront of modern molecular electronics. Electron-phonon interactions can strongly influence transport in molecular junctions. Effects attributed to these interactions include phenomena such as coherent to incoherent transition with increasing electron/vibration interaction strength, heating and heat conduction in molecular transport junctions and electronic current-induced chemical reactions.
One of the results of the interaction of electrons and phonons is the inelastic tunneling process. In the biased junction, transporting electrons can lose (or gian) a quanta of the vibration energy when qV=ℏωvib (ωvib is the frequency of a vibrational mode of the molecule), which cause a tiny change in the current. An inelastic tunneling spectroscopy (IETS) is acquired directly from the measured current-voltage (I-V) characteristics as ∂2I/∂V2 vs. V.
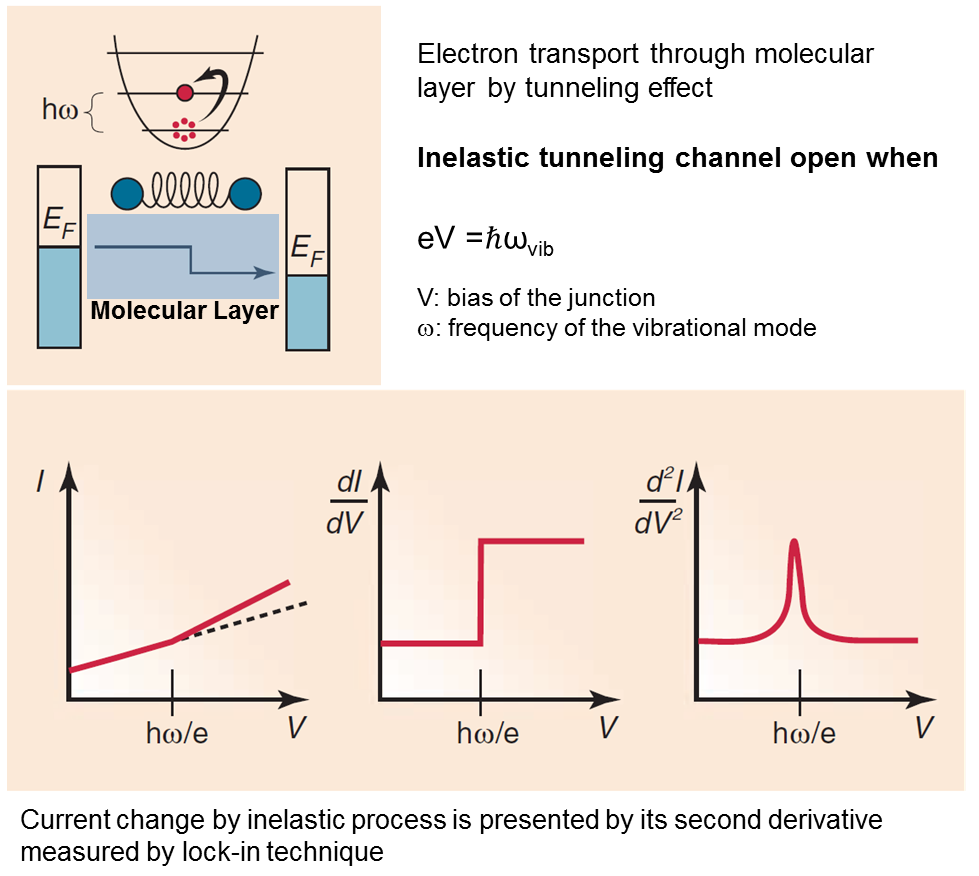
Figure from: Galperin, M.; Ratner, M. A.; Nitzan, A.; Troisi, A. Nuclear Coupling and Polarization in Molecular Transport Junctions: Beyond Tunneling to Function. Science 2008, 319, 1056-1060.
By comparing experiments and computations, IETS can confirm the actual presence of the molecules, providing information on the nature of the interfaces, on the orientation of the molecules, and on some symmetry aspects of the junction. It has been used to provide insight into molecular electronics by helping understanding electron transport mechanisms and guide design of device structures, based on electron-phonon coupling.
It is not surprising that electron-phonon interactions also play a critical role in ET via proteins, especially in the long-range tunneling process between a donor and acceptor that are parts of, or bound to a protein.
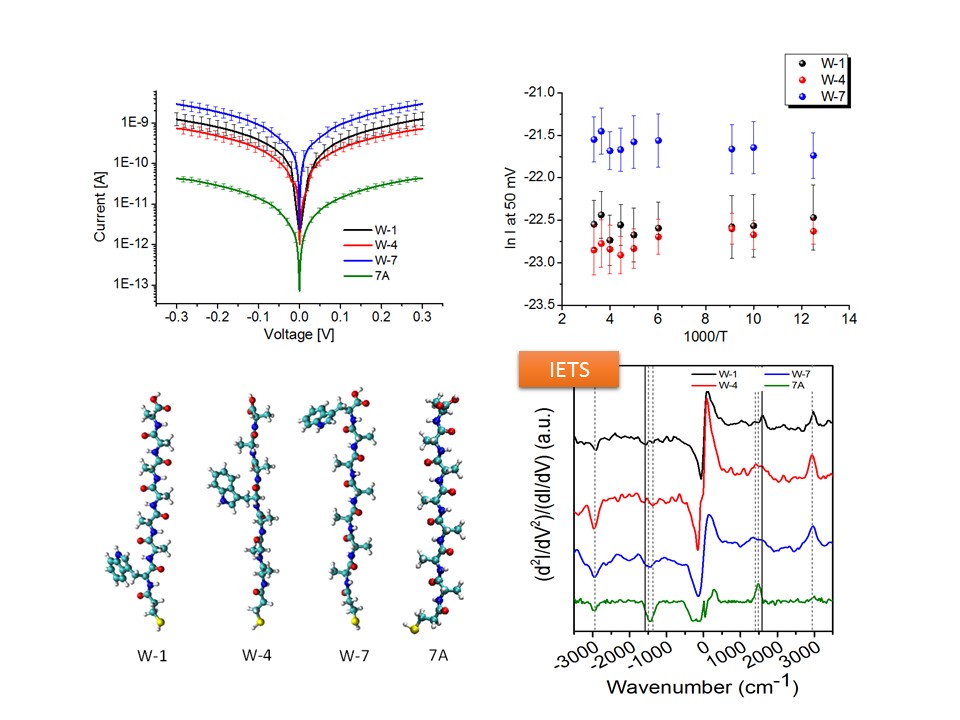
Using IETS, we explored electron-phonon interaction in metal/protein/metal junctions, to help understand solid-state electronic transport across the redox protein azurin. To that end an oriented azurin monolayer on Au is contacted by soft Au electrodes.
Characteristic vibrational modes of amide and amino acid side groups as well as of the azurin-electrode contact were observed, revealing the azurin native conformation in the junction and the critical role of side groups in the charge transport. The lack of abrupt changes in the conductance and the line shape of IETS point to far off-resonance tunneling as the dominant transport mechanism across azurin, in line with previously reported (and herein confirmed) azurin junctions. The inelastic current and hence electron-phonon interaction appear to be rather weak and comparable in magnitude with the inelastic fraction of tunneling current via alkyl chains, which may reflect the known structural rigidity of azurin.
IETS is also applied to homo- and hetero peptide junctions. The role of side group of amino acid, the amide bond, the secondary structure (etc.) in the charge transport through peptide matrix is also under investigation in our lab.
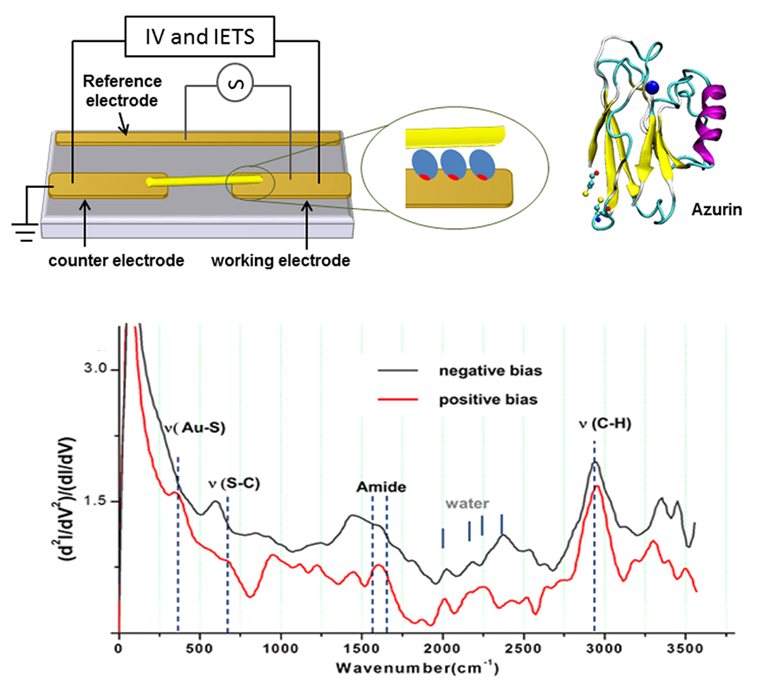
Schematic representation of azurin-based junction (top). Typical IETS spectrum of azurin (bottom).
Electron Transport via Peptides
Electron transfer (ET) via the polypeptide matrix of proteins is a key process in biological energy conversion and signaling systems. ET is sensitive to the specific sequence of amino acids and their conformation composing the protein and therefore offers a tool for chemical control of charge transport across biomaterial-based devices.
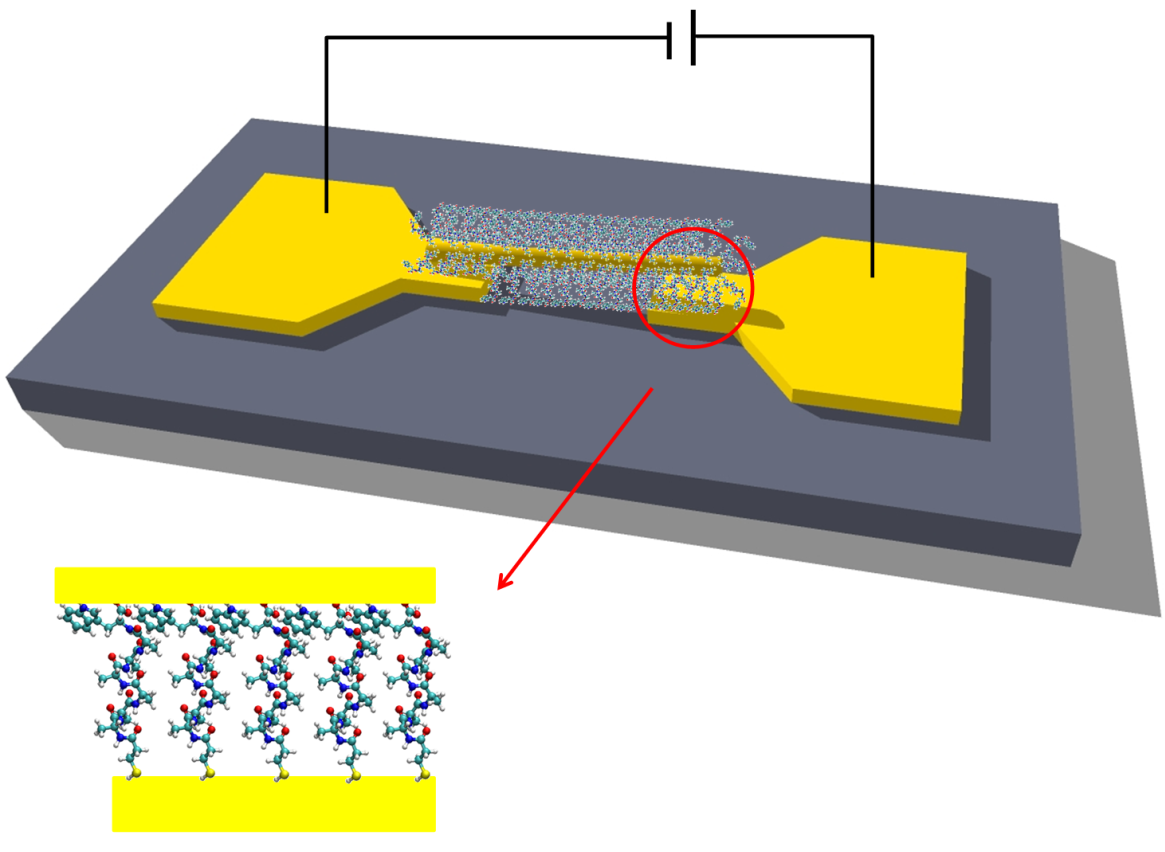
Schematic of peptide-based junction.
We studied electronic transport via several homopeptides as a function of their structural properties and temperature. We demonstrate that the conduction through the peptide depends on its length and secondary structure as well as on the nature of the constituent amino acid and charge of its residue. We support our experimental observations with high-level electronic structure calculations and suggest off-resonance tunneling as the dominant conduction mechanism via extended peptides.
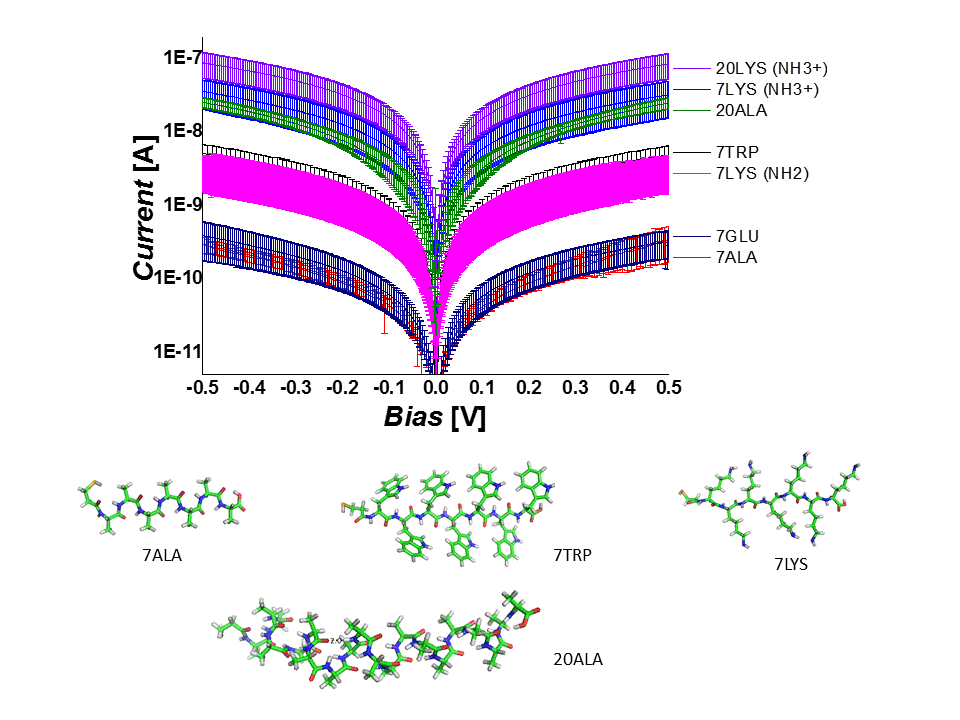
We also designed a series of hetero peptides, i. e. linear oligo-alanine peptides with a single tryptophan substitution, which acts as a “dopant”, introducing a low-lying energy level. We investigated the solid-state electron transport (ETp) across a self-assembled monolayer of these peptides between gold contacts. The single tryptophan ‘doping’ markedly increases the conductance of the peptide chain, especially when its location in the sequence is close to the electrodes. Combining inelastic tunneling spectroscopy (IETS), ultraviolet photoelectron spectroscopy (UPS), electronic structure calculations by advanced density-functional theory (DFT), and DC current-voltage analysis, the role of tryptophan in ETp is rationalized by charge tunneling across a heterogeneous energy barrier, due to electronic states of alanine and tryptophan, and by the coupling of tryptophan to the electrodes. These results reveal a controlled way of modulating the electrical properties of molecular junctions by tailor-made “building block” peptides.
Light Effect in Protein-based Junctions (CP-AFM)
The integration of the photoactive proteins with man-made electrode surfaces has attracted a great deal of interest for some two decades or more and holds significant promise from the perspective of bio(opto)electronics or energy capture interfaces. Our research focus on bacteriorhodopsin which demonstrates a light-induced structural effect. Electronic transport (ETp) in wild-type and different variants of bacteriorhodopsin has already been studied in our lab as dry patches, either in its native purple membrane environment or embedded in a pseudo-membrane. Combining the effects of the greater surface molecular density afforded by de-lipidation and the vicinity of the electrostatic changes associated with proton pumping, results in considerable enhancement of photocurrent on wavelength-selective illumination than previously achievable with multilayers of bacteriorhodopsin patches. Given the uniquely photoresponsive, wavelength-selective, and photostable characteristics of de-lipidated bacteriorhodopsin, the research has implications for utilization in solar energy capture and photodetector devices.
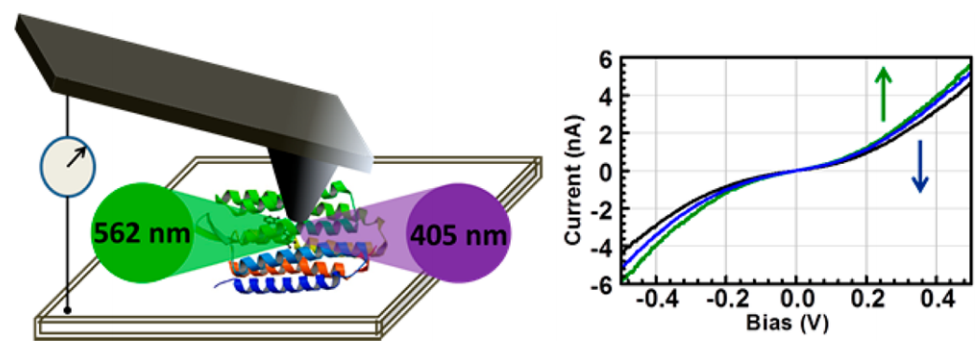
Schematic representation of light induced charge transport in de-lipidated bacteriorhodopsin in a conductive-probe AFM setup (left). Signature I-V traces for illuminated junction (right).
Electronic transport properties of native and denatured surface-immobilized proteins
Maintaining a well-defined conformation allows proteins to perform their biological function. Surprisingly, our group has found proteins to be relatively efficient solid-state electronic conductors. Properly defining their structural integrity in the dry-state is important when considering their use in future bio-electronic applications. We spectroscopically compare the properties of native and denatured proteins in a monolayer. We employ UV-vis (solution and dry state), PM-IRRAS, CD, electrochemistry and electrical measurements to piece together the influence of a protein’s secondary structure and ultimately understand the mechanisms of electronic transport in proteins.
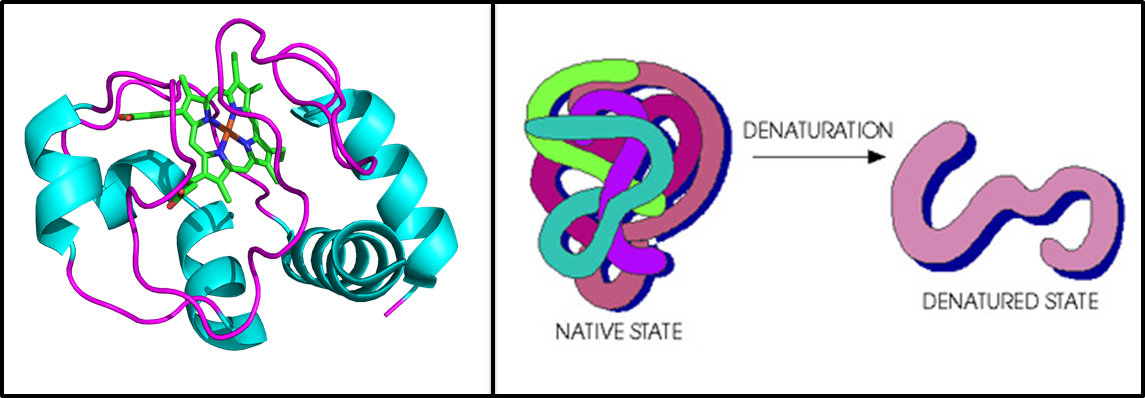
Crystal structure of horse heart cytochrome C, the test subject for this project (left). Cartoon representation of denaturation in proteins (right).


