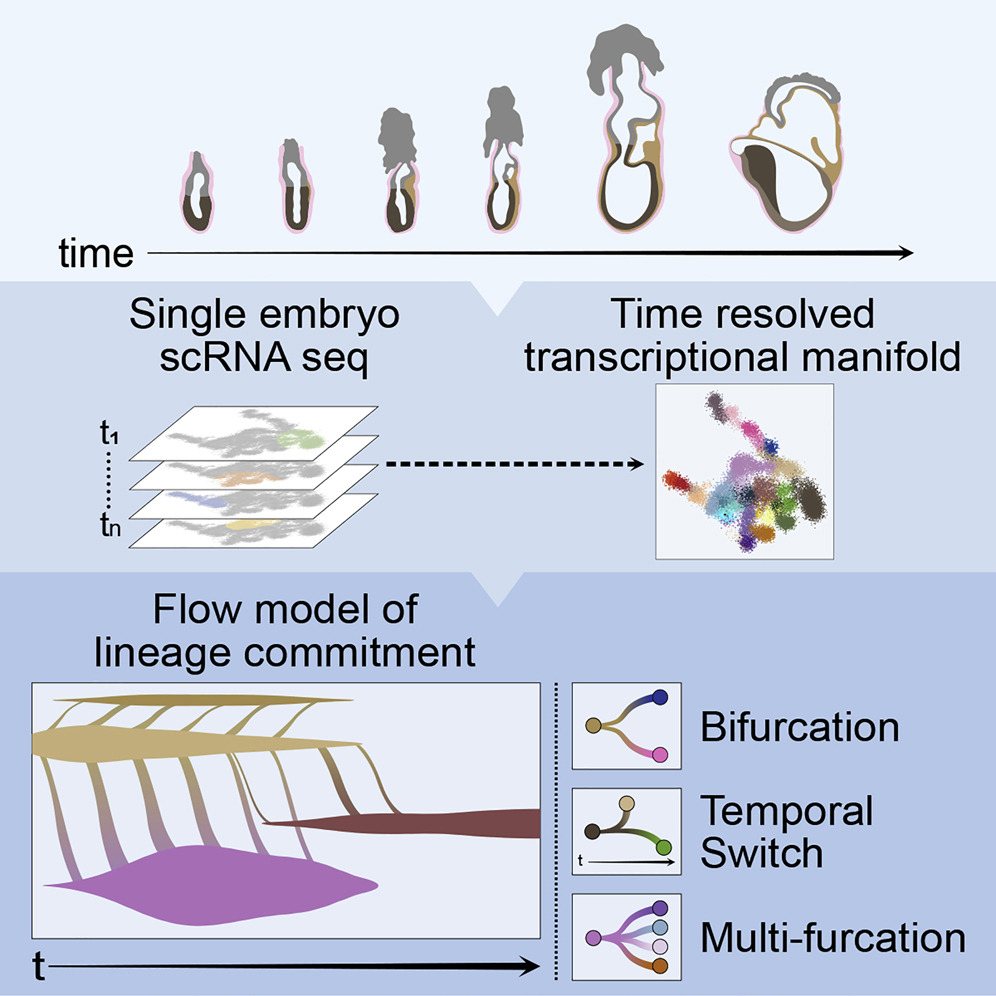
During early mammalian development, a plethora of diverse cell types arises from a single layer of epiblast cells within a short window of time. Understanding the mechanisms underlying these cell fate decisions is challenging because of the interplay of various intrinsic (transcription factors, epigenetic changes) and extrinsic (signaling molecules) factors. We recently introduced a new temporal model of mouse gastrulation, that allows us to infer precise differentiation flows and lineage specification dynamics for every final transcriptional state. Current work focuses on understanding the regulation of gene expression changes. To this end we are
- using in vitro models of gastrulation to study perturbations of signaling factors
- connecting gene expression dynamics with epigenetic changes such as enhancer accessibilities (scATAC + scRNAseq), DNA methylation dynamics or chromosome conformation dynamics (Hi-C).