Publications
-
(2024) Developmental Biology and Cancer. 1st ed. Boca Raton: . p. 211-234 Abstract
The relationship between cell shape and cell function is one of the central themes in cell biology. Cell form is used by cytologists as a marker for defining the tissue of origin and the physiological status of cells cultured in vitro. Cell shape is determined in a large part by mechanical forces generated by cellcell and cell-extracellular-matrix (ECM) contacts and by the cytoskeleton (for reviews see Harris, 1987; Ingber, 1991).
-
(2024) Cells. 13, 21, 1810. Abstract
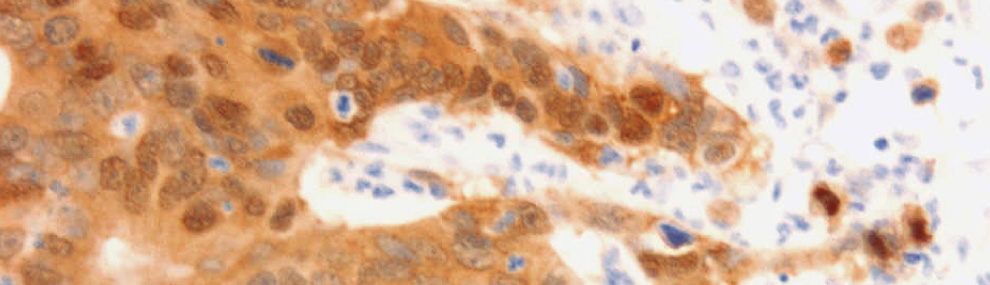
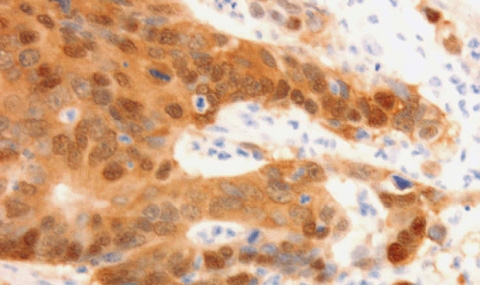
The cell adhesion molecule L1CAM (L1), mainly known for its function in brain cells, is a Wnt/β-catenin signaling target gene in colorectal cancer (CRC) cells, where it promotes invasion and liver metastasis. We interrogated which genes are expressed at increased levels in human CRC tissue and induced in CRC cell lines overexpressing L1. We found increased cyclin D2 levels in CRC tissue and LS 174T and HCT 116 human CRC cells overexpressing L1. Increased cyclin D2 in CRC cells was associated with higher proliferation rates, faster motility, tumorigenesis, and liver metastasis. The suppression of cyclin D2 expression by shRNA to cyclin D2 blocked the increase in these cellular properties of L1-expressing cells. The overexpression of cyclin D2 in the absence of L1 also conferred tumorigenic properties similar to L1 expression. The pathways involved in the elevation of cyclin D2 by L1 include NF-κB, Akt, and β-catenin signaling but not the Erk pathway. We found that in a significant percentage of human CRC tissue samples, cyclin D2 is expressed at high levels in the nuclei of cancer cells. At the same time, the adjacent normal mucosa was negative for cyclin D2 staining. The results suggest that the increased cyclin D2 expression by L1 is required to induce proliferative, motile tumor development in CRC tissue and can serve as a diagnostic marker and a target for CRC therapy.
-
(2023) International Journal of Molecular Sciences. 24, 17, 13418. Abstract
An induction in the expression of the cell adhesion receptor L1, a Wnt target gene, is a characteristic feature of Wnt/β-catenin activation in colon cancer cells at later stages of the disease. We investigated the proteins secreted following L1 expression in colon cancer cells and identified Mucin2 among the most abundant secreted proteins. We found that suppressing Mucin2 expression in L1-expressing colon cancer cells inhibits cell proliferation, motility, tumorigenesis, and liver metastasis. We detected several signaling pathways involved in Mucin2 induction in L1-expressing cells. In human colon cancer tissue, Mucin2 expression was significantly reduced or lost in the adenocarcinoma tissue, while in the mucinous subtype of colon cancer tissue, Mucin2 expression was increased. An increased signature of L1/Mucin2 expression reduced the survival rate of human colon cancer patients. Thus, induction of Mucin2 expression by L1 is required during mucinous colon cancer progression and can serve as a marker for diagnosis and a target for therapy.
-
(2022) Cancers. 14, 18, 4478. Abstract
The immunoglobulin family cell adhesion receptor L1 is induced in CRC cells at the invasive front of the tumor tissue, and confers enhanced proliferation, motility, tumorigenesis, and liver metastasis. To identify putative tumor suppressors whose expression is downregulated in L1-expressing CRC cells, we blocked the L1ezrinNF-κB signaling pathway and searched for genes induced under these conditions. We found that TFF1, a protein involved in protecting the mucus epithelial layer of the colon, is downregulated in L1-expressing cells and displays characteristics of a tumor suppressor. Overexpression of TFF1 in L1-transfected human CRC cells blocks the pro-tumorigenic and metastatic properties conferred by L1 by suppressing NF-κB signaling. Immunohistochemical analyses revealed that human CRC tissue samples often lose the expression of TFF1, while the normal mucosa displays TFF1 in goblet cells. Identifying TFF1 as a tumor suppressor in CRC cells could provide a novel marker for L1-mediated CRC development and a potential target for therapy.
-
(2022) International Journal of Molecular Sciences. 23, 1, 445. Abstract
Aberrant activation of Wnt/βcatenin signaling and downstream βcateninTCF target genes is a hallmark of colorectal cancer (CRC) development. We identified the immunoglobulinlike cell adhesion receptor L1CAM (L1) as a target of βcateninTCF transactivation in CRC cells. Overexpression of L1 in CRC cells confers enhanced proliferation, motility, tumorigenesis, and liver metastasis, and L1 is exclusively localized at invasive areas of human CRC tissue. Several genes are induced after L1 transfection into CRC cells by a mechanism involving the L1ezrinNFκB pathway. We conducted a secretomic analysis of the proteins in the culture medium of L1overexpressing CRC cells. We detected a highly increased level of biglycan, a small leucinerich ECM component, and a signaling molecule. We found that induction of biglycan is required for the cellular processes conferred by L1, including enhanced proliferation, motility, tumorigenesis, and liver metastasis. The suppression of endogenous biglycan levels or a point mutation in the L1 ectodomain that regulates cellcell adhesion mediated by L1 blocked the enhanced tumorigenic properties conferred by L1. The mechanism of biglycan induction by L1 involves the L1NFκB pathway. Blocking NFκB signaling in L1 expressing cells suppressed the induction of biglycan and the tumorigenic properties conferred by L1. Biglycan expression was undetectable in the normal colonic mucosa, but expressed at highly increased levels in the tumor tissue, especially in the stroma. The therapeutic strategies to target biglycan expression might provide a useful approach for CRC treatment in L1overexpressing tumors.
-
(2021) International Journal of Molecular Sciences. 22, 7, 3552. Abstract
The overactivation of Wnt/βcatenin signaling is a hallmark of colorectal cancer (CRC) development. We identified the cell adhesion molecule L1CAM (L1) as a target of βcateninTCF transactivation in CRC cells. The overexpression of L1 in CRC cells confers enhanced proliferation, motility, tumorigenesis and liver metastasis, and L1 is exclusively localized in the invasive areas of human CRC tissue. A number of genes are induced after L1 transfection into CRC cells by a mechanism involving the cytoskeletal protein ezrin and the NFĸB pathway. When studying the changes in gene expression in CRC cells overexpressing L1 in which ezrin levels were suppressed by shRNA to ezrin, we discovered the collagenmodifying enzyme lysyl hydroxylase 2 (PLOD2) among these genes. We found that increased PLOD2 expression was required for the cellular processes conferred by L1, including enhanced proliferation, motility, tumorigenesis and liver metastasis, since the suppression of endogenous PLOD2 expression, or its enzymatic activity, blocked the enhanced tumor-igenic properties conferred by L1. The mechanism involved in increased PLOD2 expression by L1 involves ezrin signaling and PLOD2 that affect the SMAD2/3 pathway. We found that PLOD2 is localized in the colonic crypts in the stem cell compartment of the normal mucosa and is found at increased levels in invasive areas of the tumor and, in some cases, throughout the tumor tissue. The therapeutic strategies to target PLOD2 expression might provide a useful approach for CRC treat-ment.
-
(2020) International Journal of Cancer. 147, 12, p. 3292-3296 Abstract
First described as a neuronal cell adhesion molecule, L1CAM was later identified to be present at increased levels in primary tumors and metastases of various types of cancer. Here, we describe the multifaceted roles of L1CAM that are involved in diverse fundamental steps during tumor initiation and progression, as well as in chemoresistance. Recently, Ganesh et al reported that L1CAM identifies metastasis-initiating cells in colorectal carcinoma exhibiting stem-like cell features, increased tumorigenic potential and enhanced chemoresistance. In this review, we highlight recent advances in L1CAM research with particular emphasis on its role in de-differentiation processes and cancer cell stemness supporting the view that L1CAM is a powerful prognostic factor and a suitable target for improved therapy of metastatic and drug-resistant tumors.
-
(2020) Cancers. 12, 11, p. 1-13 3444. Abstract
Cell adhesion to neighboring cells is a fundamental biological process in multicellular organisms that is required for tissue morphogenesis. A tight coordination between cellcell adhesion, signaling, and gene expression is a characteristic feature of normal tissues. Changes, and often disruption of this coordination, are common during invasive and metastatic cancer development. The Wnt/β-catenin signaling pathway is an excellent model for studying the role of adhesion-mediated signaling in colorectal cancer (CRC) invasion and metastasis, because β-catenin has a dual role in the cell; it is a major adhesion linker of cadherin transmembrane receptors to the cytoskeleton and, in addition, it is also a key transducer of Wnt signaling to the nucleus, where it acts as a co-transcriptional activator of Wnt target genes. Hyperactivation of Wnt/β-catenin signaling is a common feature in the majority of CRC patients. We found that the neural cell adhesion receptor L1CAM (L1) is a target gene of β-catenin signaling and is induced in carcinoma cells of CRC patients, where it plays an important role in CRC metastasis. In this review, we will discuss studies on β-catenin target genes activated during CRC development (in particular, L1), the signaling pathways affected by L1, and the role of downstream target genes activated by L1 overexpression, especially those that are also part of the intestinal stem cell gene signature. As intestinal stem cells are highly regulated by Wnt signaling and are believed to also play major roles in CRC progression, unravelling the mechanisms underlying the regulation of these genes will shed light on both normal intestinal homeostasis and the development of invasive and metastatic CRC.
-
(2019) Oncotarget. 10, 67, p. 7122-7131 Abstract
Hyperactivation of Wnt/β-catenin target gene expression is a hallmark of colorectal cancer (CRC) development. We identified L1-CAM (L1) and Nr-CAM, members of the immunoglobulin family of nerve cell adhesion receptors, as target genes of the Wnt/β-catenin pathway in CRC cells. L1 overexpression in CRC cells enhances their motile and tumorigenic capacity and promotes liver metastasis. L1 is often localized at the invasive edge of CRC tissue. Using gene arrays and proteomic analyses we identified downstream signaling pathways and targets of L1-mediated signaling. Here, we found that the expression of interferon-stimulated gene 15 (ISG15) that operates much like ubiquitin (is conjugated to proteins by ISGylation), is elevated in the conditioned medium and in CRC cells overexpressing L1. Suppression of endogenous ISG15 levels in L1-expressing cells blocked the increased proliferative, motile, tumorigenic and liver metastatic capacities of CRC cells. ISG15 overexpression, on its own, could enhance these properties in CRC cells, but only to a much lower extent compared to L1. We show that NF-κB signaling is involved in the L1-mediated increase in ISG15, since blocking the NF-κB pathway abolished the induction of ISG15 by L1. Point mutations in the L1 ectodomain that interfere with its binding to L1 ligands, also inhibited the increase in ISG15. We detected high levels of ISG15 in human CRC tissue cells and in the adjacent stroma, but not in the normal mucosa. The results suggest that ISG15 is involved in L1-mediated CRC development and is a potential target for CRC therapy.
-
(2019) Oncotarget. 10, 50, p. 5217-5228 Abstract
Hyperactivation of Wnt/β-catenin target genes is considered a key step in human colorectal cancer (CRC) development. We previously identified the immunoglobulin-like cell adhesion receptor L1 as a target gene of β-catenin/TCF transactivation that is localized at the invasive edge of CRC tissue. Using gene arrays, we discovered a number of downstream target genes and signaling pathways conferred by L1 overexpression during colon cancer progression. Here, we have used a proteomic approach to identify proteins in the secretome of L1-overexpressing CRC cells and studied the role of the increase in the aspartate protease cathepsin D (CTSD) in L1-mediated colon cancer development. We found that in addition to the increase in CTSD in the secretome, the RNA and protein levels of CTSD were also induced by L1 in CRC cells. CTSD overexpression resulted in elevated proliferation under stress and increased motility, tumorigenesis and liver metastasis, although to a lesser extent than after L1-transfection. The suppression of endogenous CTSD in L1-expressing cells blocked the increase in the proliferative, motile, tumorigenic and metastatic ability of CRC cells. Enhancing Wnt/β-catenin signaling by the inhibition of GSK3β resulted in increased endogenous CTSD levels, suggesting the involvement of the Wnt/β-catenin pathway in CTSD expression. In human CRC tissue, CTSD was detected in epithelial cells and in the stromal compartment at the more invasive areas of the tumor, but not in the normal mucosa, indicating that CTSD plays an essential role in CRC progression.
-
(2018) Cancer Letters. 424, p. 9-18 Abstract
Aberrant Wnt/beta-catenin signaling is a common event during human colorectal cancer (CRC) development. Previously, we characterized members of the L1 family of cell adhesion receptors as targets of beta-catenin-LEF1/TCF transactivation that are expressed at the invasive CRC tissue edge. Overexpression of L1 in CRC cells confers enhanced motility, tumorigenesis and liver metastasis. We identified several downstream targets of Ll-mediated signaling that are considered key intestinal stem cell signature genes. Here, we investigated the involvement of ASCL2, a Wnt target gene and key determinant of intestinal stem cell state, in L1-mediated CRC progression. In L1 overexpressing CRC cells we found an increase in ASCL2, a decrease in E-cadherin and accumulation of nuclear beta-catenin, beta-catenin-LEFliTCF transactivation and target gene expression. The increase in ASCL2 by L1 overexpression enhanced ASCL2 target gene expression, conferred increased motility, tumorigenesis and metastasis, similar to L1 overexpression. Suppression of ASCL2 in cells expressing L1 blocked these tumorigenic properties. In human CRC tissue, ASCL2 was detected in the nuclei of cells at invasive areas of the tumor that also expressed L1. The results suggest that increased ASCL2 expression is a critical step in L1-mediated CRC progression. (C) 2018 Elsevier B.V. All rights reserved.
-
(2018) F1000Research. 7, 1488. Abstract
Changes in cell adhesion and motility are considered key elements in determining the development of invasive and metastatic tumors. Co-opting the epithelial-to-mesenchymal transition (EMT) process, which is known to occur during embryonic development, and the associated changes in cell adhesion properties in cancer cells are considered major routes for tumor progression. More recent in vivo studies in tumor tissues and circulating tumor cell clusters suggest a stepwise EMT process rather than an "all-or-none" transition during tumor progression. In this commentary, we addressed the molecular mechanisms underlying the changes in cell adhesion and motility and adhesion-mediated signaling and their relationships to the partial EMT states and the acquisition of stemness traits by cancer cells.
-
(2017) Oncogene. 36, 11, p. 1597-1606 Abstract
The neural L1 transmembrane cell adhesion receptor of the immunoglobulin-like family is a target gene of Wnt-beta-catenin signaling in human colorectal cancer (CRC) cells and is expressed at the invasive edge of the tumor tissue. L1 overexpression in cultured CRC cells confers enhanced proliferation, motility and liver metastasis. We have analyzed the mechanisms of L1-mediated signaling in CRC cells by using various point mutations in the L1 ectodomain that are known to cause severe genetically inherited mental retardation disorders in patients. We found that all such L1 ectodomain mutations abolish the ability of L1 to confer metastatic properties in CRC cells. Using gene array analysis, we identified L1-mutation-specific gene expression signatures for the L1/H210Q and L1/D598N mutations. We identified CD10, a metalloprotease (neprilysin, neutral endopeptidase) and a gene that is specifically induced in CRC cells by L1 in an L1/H210Q mutation-specific manner. CD10 expression was required for the L1-mediated induction of cell proliferation, motility and metastasis, as suppression of CD10 levels in L1-expressing CRC cells abolished the L1 effects on CRC progression. The signaling from L1 to CD10 was mediated through the L1-ezrin-NF-.B pathway. In human CRC tissue L1 and CD10 were localized in partially overlapping regions in the more invasive areas of the tumor tissue. The results suggest that CD10 is a necessary component conferring the L1 effects in CRC cells. The identification of gene expression patterns of L1-domain-specific point mutations may provide novel markers and targets for interfering with L1-mediated CRC progression.
-
(2016) Cancers. 8, 5, 27187476. Abstract
The Wnt-beta-catenin signaling pathway is highly conserved during evolution and determines normal tissue homeostasis. Hyperactivation of Wnt--catenin signaling is a characteristic feature of colorectal cancer (CRC) development. -catenin is a major transducer of the Wnt signal from the cytoplasm into the nucleus where it acts as a co-transcriptional activator of -catenin-TCF target genes. beta-catenin is also required for linking cadherin type cell-cell adhesion receptors to the cytoskeleton, and consequently Wnt-beta-catenin signaling is an attractive system for investigating the role of adhesion-mediated signaling in both normal intestinal tissue homeostasis and CRC development. In this review, we summarize our studies on one Wnt-beta-catenin target gene, L1, a member of the immunoglobulin-like cell adhesion transmembrane receptor family. We describe the mechanisms of L1-mediated signaling in CRC cells, its exclusive localization in invasive areas of CRC tissue, and its ability to increase cell motility and confer metastasis to the liver. We discuss the activation (by L1) of genes via an ezrin-NF-kappa B pathway and the induction of genes also found in the intestinal stem cell signature. By studying L1 (adhesion)-mediated signaling, we expect to learn about mechanisms regulating both normal intestinal homeostasis and CRC development.
-
(2016) F1000Research. 5, 699. Abstract
Overactivation of Wnt signaling is a hallmark of colorectal cancer (CRC). The Wnt pathway is a key regulator of both the early and the later, more invasive, stages of CRC development. In the normal intestine and colon, Wnt signaling controls the homeostasis of intestinal stem cells (ISCs) that fuel, via proliferation, upward movement of progeny cells from the crypt bottom toward the villus and differentiation into all cell types that constitute the intestine. Studies in recent years suggested that cancer stem cells (CSCs), similar to ISCs of the crypts, consist of a small subpopulation of the tumor and are responsible for the initiation and progression of the disease. Although various ISC signature genes were also identified as CRC markers and some of these genes were even demonstrated to have a direct functional role in CRC development, the origin of CSCs and their contribution to cancer progression is still debated. Here, we describe studies supporting a relationship between Wnt-regulated CSCs and the progression of CRC.
-
-
(2015) Oncogene. 35, 5, p. 549-557 Abstract
Overactivation of Wnt-β-catenin signaling, including β-catenin-TCF target gene expression, is a hallmark of colorectal cancer (CRC) development. We identified the immunoglobulin family of cell-adhesion receptors member L1 as a β-catenin-TCF target gene preferentially expressed at the invasive edge of human CRC tissue. L1 can confer enhanced motility and liver metastasis when expressed in CRC cells. This ability of L1-mediated metastasis is exerted by a mechanism involving ezrin and the activation of NF-κB target genes. In this study, we identified the secreted modular calcium-binding matricellular protein-2 (SMOC-2) as a gene activated by L1-ezrin-NF-κB signaling. SMOC-2 is also known as an intestinal stem cell signature gene in mice expressing Lgr5 in cells at the bottom of intestinal crypts. The induction of SMOC-2 expression in L1-expressing CRC cells was necessary for the increase in cell motility, proliferation under stress and liver metastasis conferred by L1. SMOC-2 expression induced a more mesenchymal like phenotype in CRC cells, a decrease in E-cadherin and an increase in Snail by signaling that involves integrin-linked kinase (ILK). SMOC-2 was localized at the bottom of normal human colonic crypts and at increased levels in CRC tissue with preferential expression in invasive areas of the tumor. We found an increase in Lgr5 levels in CRC cells overexpressing L1, p65 or SMOC-2, suggesting that L1-mediated CRC progression involves the acquisition of a stem cell-like phenotype, and that SMOC-2 elevation is necessary for L1-mediated induction of more aggressive/invasive CRC properties.
-
(2015) Oncotarget. 6, 33, p. 34389-34401 Abstract
Hyperactive Wnt signaling is a common feature in human colorectal cancer (CRC) cells. A central question is the identification and role of Wnt/β-catenin target genes in CRC and their relationship to genes enriched in colonic stem cells, since Lgr5+ intestinal stem cells were suggested to be the cell of CRC origin. Previously, we identified the neural immunoglobulin-like adhesion receptor L1 as a Wnt/β-catenin target gene localized in cells at the invasive front of CRC tissue and showed that L1 expression in CRC cells confers enhanced motility and liver metastasis. Here, we identified the clusterin (CLU) gene that is also enriched in Lgr5+ intestinal stem cells, as a gene induced during L1-mediated CRC metastasis. The increase in CLU levels by L1 in CRC cells resulted from transactivation of CLU by STAT-1. CLU overexpression in CRC cells enhanced their motility and the reduction in CLU levels in L1 overexpressing cells suppressed the ability of L1 to confer increased tumorigenesis and liver metastasis. Genes induced during L1-mediated CRC cell metastasis and enriched in intestinal stem cells might be important for both CRC progression and colonic epithelium homeostasis.
-
(2014) Journal of General Physiology. 143, 5, p. 645-656 Abstract
The basic carboxy terminus of p53 plays an important role in directing the protein into the nuclear compartment. The C terminus of the p53 molecule contains a cluster of several nuclear localization signals (NLSs) that mediate the migration of the protein into the cell nucleus. NLSI, the most active domain, is highly conserved in genetically diverged species and shares perfect homology with consensus NLS sequences found in other nuclear proteins. The other two NLSs, II and III, appear to be less effective and less conserved. Although nuclear localization is dictated primarily by the NLSs inherent in the primary amino acid sequence, the actual nuclear homing can be modified by interactions with other proteins expressed in the cell. Comparison between wild-type p53 and naturally occurring mutant p53 showed that both protein categories could migrate into the nucleus of rat primary embryonic fibroblasts by essentially similar mechanisms. Nuclear localization of both proteins was totally dependent on the existence of functional NLS domains. In COS cells, however, we found that NLS-deprived wild-type p53 molecules could migrate into the nucleus by complexing with another nuclear protein, simian virus 40 large-T antigen. Wild-type and mutant p53 proteins differentially complexed with viral or cellular proteins, which may significantly affect the ultimate compartmentalization of p53 in the cell; this finding suggests that the actual subcellular compartmentalization of proteins may difer in various cell type milieux and may largely be affected by the ability of these proteins to complex with other proteins expressed in the cell. Experiments designed to test the physiological significance of p53 subcellular localization indicated that nuclear localization of mutant p53 is essential for this protein to enhance the process of malignant transformation of partially transformed cells, suggesting that p53 functions within the cell nucleus.
-
(2013) Cancer Research. 73, 18, p. 5754-5763 Abstract
The transmembrane neural cell adhesion receptor L1 is a Wnt/b-catenin target gene expressed in many tumor types. In human colorectal cancer, L1 localizes preferentially to the invasive front of tumors and when overexpressed in colorectal cancer cells, it facilitates their metastasis to the liver. In this study, we investigated genes that are regulated in human colorectal cancer and by the L1-NF-kB pathway that has been implicated in liver metastasis. c-Kit was the most highly suppressed gene in both colorectal cancer tissue and the L1-NF-kB pathway. c-Kit suppression that resulted from L1-mediated signaling relied upon NF-kB, which directly inhibited the transcription of SP1, a major activator of the c-Kit gene promoter. Reconstituting c-Kit expression in L1- transfected cells blocked the biological effects conferred by L1 overexpression in driving motility and liver metastasis. We found that c-Kit expression in colorectal cancer cells is associated with a more pronounced epithelial morphology, along with increased expression of E-cadherin and decreased expression of Slug. Although c-Kit overexpression inhibited the motility and metastasis of L1-expressing colorectal cancer cells, it enhanced colorectal cancer cell proliferation and tumorigenesis, arguing that separate pathways mediate tumorigenicity and metastasis by c-Kit. Our findings provide insights into how colorectal cancer metastasizes to the liver, the most common site of dissemination in this cancer.
-
(2013) Oncogene. 32, 27, p. 3220-3230 Abstract
L1, a neuronal cell adhesion receptor of the immunoglobulin-like protein family is expressed in invading colorectal cancer (CRC) cells as a target gene of Wnt/β-catenin signaling. Overexpression of L1 in CRC cells enhances cell motility and proliferation, and confers liver metastasis. We recently identified ezrin and the IκB-NF-κB pathway as essential for the biological properties conferred by L1 in CRC cells. Here, we studied the underlying molecular mechanisms and found that L1 enhances ezrin phosphorylation, via Rho-associated protein kinase (ROCK), and is required for L1-ezrin co-localization at the juxtamembrane domain and for enhancing cell motility. Global transcriptomes from L1-expressing CRC cells were compared with transcriptomes from the same cells expressing small hairpin RNA (shRNA) to ezrin. Among the genes whose expression was elevated by L1 and ezrin we identified insulin-like growth factor-binding protein 2 (IGFBP-2) and showed that its increased expression is mediated by an NF-κB-mediated transactivation of the IGFBP-2 gene promoter. Expression of a constitutively activated mutant ezrin (Ezrin567D) could also increase IGFBP-2 levels in CRC cells. Overexpression of IGFBP-2 in CRC cells lacking L1-enhanced cell proliferation (in the absence of serum), cell motility, tumorigenesis and induced liver metastasis, similar to L1 overexpression. Suppression of endogenous IGFBP-2 in L1-transfected cells inhibited these properties conferred by L1. We detected IGFBP-2 in a unique organization at the bottom of human colonic crypts in normal mucosa and at increased levels throughout human CRC tissue samples co-localizing with the phosphorylated p65 subunit of NF-κB. Finally, we found that IGFBP-2 and L1 can form a molecular complex suggesting that L1-mediated signaling by the L1-ezrin-NF-κB pathway, that induces IGFBP-2 expression, has an important role in CRC progression.
-
How to unravel the secrets of cancer metastasis?(2012) Galileo. p. 20-23 Abstract
בזמן התפתחות עוּברית,מתחלקים התאים יותרמטריליון פעם, ביוצרם,בדיוק מופלא, את הרקמות והאיברים השונים שמרכיבים את גוף האדם הבוגר. כשחלוקה זו יוצאת מכלל שליטה, מתפתחת מחלת הסרטן, שבהמשך וצרת גרורות של תאים סרטניים בגוף האדם. איך אפשר להילחם בהן?
-
(2011) Molecular Cancer Research. 9, 1, p. 14-24 Abstract
Aberrant activation of Wnt/β-catenin signaling is common in most sporadic and inherited colorectal cancer (CRC) cells leading to elevated β-catenin/TCF transactivation. We previously identified the neural cell adhesion molecule L1 as a target gene of β-catenin/TCF in CRC cells. Forced expression of L1 confers increased cell motility, invasion, and tumorigenesis, and the induction of human CRC cell metastasis to the liver. In human CRC tissue, L1 is exclusively localized at the invasive front of such tumors in a subpopulation of cells displaying nuclear β-catenin. We determined whether L1 expression confers metastatic capacities by inducing an epithelial to mesenchymal transition (EMT) and whether L1 cosegregates with cancer stem cell (CSC) markers. We found that changes in L1 levels do not affect the organization or expression of E-cadherin in cell lines, or in invading CRC tissue cells, and no changes in other epithelial or mesenchymal markers were detected after L1 transfection. The introduction of major EMT regulators (Slug and Twist) into CRC cell lines reduced the levels of E-cadherin and induced fibronectin and vimentin, but unlike L1, Slug and Twist expression was insufficient for conferring metastasis. In CRC cells L1 did not specifically cosegregate with CSC markers including CD133, CD44, and EpCAM. L1-mediated metastasis required NF-κB signaling in cells harboring either high or low levels of endogenous E-cadherin. The results suggest that L1-mediated metastasis of CRC cells does not require changes in EMT and CSC markers and operates by activating NF-κβ signaling.
-
(2010) F1000 Biology Reports. 2, 1, 86. Abstract
Understanding the progression of a primary cancer to themetastatic stage has been the focus of extensive research for years. Commonly accepted concepts in this process (i.e., that of genetic instability and loss of normal cellular constraints on growth and motility) are well established. Other important paradigms, such as the necessary change from an epithelial cell phenotype displaying cell-cell adhesions to a singular and motile mesenchymal-like cell phenotype (possibly derived from a stem cell-like cell) via a process similar to epithelial to mesenchymal transition (EMT), are less well understood. In this review we will address studies linking EMT and cancer stem cells during cancer development and observations that are challenging these concepts.
-
-
-
Nuclear factor-κB signaling and ezrin are essential for L1-mediated metastasis of colon cancer cells(2010) Journal of Cell Science. 123, 12, p. 2135-2143 Abstract
Hyperactivation of β-catenin-T-cell-factor (TCF)-regulated gene transcription is a hallmark of colorectal cancer (CRC). The cell-neural adhesion molecule L1CAM (hereafter referred to as L1) is a target of β-catenin-TCF, exclusively expressed at the CRC invasive front in humans. L1 overexpression in CRC cells increases cell growth and motility, and promotes liver metastasis. Genes induced by L1 are also expressed in human CRC tissue but the mechanisms by which L1 confers metastasis are still unknown. We found that signaling by the nuclear factor κB (NF-κB) is essential, because inhibition of signaling by the inhibitor of κB super repressor (IκB-SR) blocked L1-mediated metastasis. Overexpression of the NF-κB p65 subunit was sufficient to increase CRC cell proliferation, motility and metastasis. Binding of the L1 cytodomain to ezrin - a cytoskeleton-crosslinking protein - is necessary for metastasis because when binding to L1 was interrupted or ezrin gene expression was suppressed with specific shRNA, metastasis did not occur. L1 and ezrin bound to and mediated the phosphorylation of IκB. We also observed a complex containing IκB, L1 and ezrin in the juxtamembrane region of CRC cells. Furthermore, we found that L1, ezrin and phosphorylated p65 are co-expressed at the invasive front in human CRC tissue, indicating that L1-mediated activation of NF-κB signaling involving ezrin is a major route of CRC progression.
-
(2009) Cancer Letters. 282, 2, p. 137-145 Abstract
The L1 cell adhesion molecule (L1CAM) belongs to the immunoglobulin superfamily and was originally identified in the nervous system. Recent studies demonstrated L1CAM expression in various types of cancer, predominantly at the invasive front of tumors and in metastases, suggesting its involvement in advanced stages of tumor progression. Overexpression of L1CAM in normal and cancer cells increased motility, enhanced growth rate and promoted cell transformation and tumorigenicity. Moreover, the expression of L1CAM in tumor cells conferred the capacity to form metastases. These properties of L1CAM, in addition to its cell surface localization, make it a potentially useful diagnostic marker for cancer progression and a candidate for anti-cancer therapy. We review the role of L1CAM in cancer progression with particular emphasis on colon cancer, and the potential of anti-L1CAM antibodies as a therapeutic tool for cancer.
-
(2009) Journal of Cellular Biochemistry. 108, 1, p. 326-336 Abstract
A key step in human colon cancer development includes the hyperactivation of Wnt/β-catenin signaling and the induction of β-catenin-TCF target genes that participate in colon cancer progression. Recent studies identified members of the immunoglobulin-like cell adhesion molecules (IgCAM) of the L1CAM family (L1 and Nr-CAM) as targets of β-catenin-TCF signaling in colon cancer cells. L1 was detected at the invasive front of colon cancer tissue and confers metastasis when overexpressed in cells. In contrast to L1, we did not detect in colon cancer cells significant levels of another IgCAM family of molecules, the nectin-like (Necl) receptors Necl1 and Necl4, while Necl4 was previously found in the normal small intestine and colon tissues. We studied the properties of colon cancer cells in which Necl4 and Necl1 were expressed either alone, or in combination, and found that such cells display a wide range of properties associated with tumor suppression. Expression of both Necl1 and Necl4 was the most efficient in suppressing the tumorigenicity of colon cancer cells. This was associated with enhanced rates of apoptosis and change in several apoptosis-related markers. In contrast to its capacity to suppress tumorigenesis, Necl4 was unable to affect the highly malignant and metastatic capacities of colon cancer cells in which L1 was overexpressed. Our results suggest that various IgCAM receptor families play different roles in affecting the tumorigenic function of the same cells, and that Necl1 and Necl4 can fulfill a tumor suppressive role.
-
(2008) Expert Opinion on Biological Therapy. 8, 11, p. 1749-1757 Abstract
Background: L1-cell adhesion molecule (L1-CAM) is a cell adhesion receptor of the immunoglobulin superfamily, known for its roles in nerve cell function. While originally believed to be present only in brain cells, in recent years L1-CAM has been detected in other tissues, and in a variety of cancer cells, including some common types of human cancer. Objective/methods: We review the prevalence of L1-CAM in human cancer, the possible mechanisms involved in L1-CAM-mediated tumorigenesis, and cancer therapies based upon L1-CAM antibody treatment. Results/conclusions: In colon cancer cells, the L1-CAM gene was identified as a target of the Wnt/β-catenin-TCF signaling pathway, and L1-CAM was exclusively detected at the invasive front of colon and ovarian cancer tissue. The expression of L1-CAM in normal and cancer cells enhanced tumorigenesis and conferred metastasis in colon cancer cells. Antibodies against the L1-CAM ectodomain severely inhibited the proliferation of a variety of cancer cells in culture and reduced tumor burden when injected into mice harboring cancer cells expressing L1-CAM. These results, in addition to the presence of L1-CAM on the cell surface and its restricted distribution in normal tissues, make it an ideal target for tumor therapy.
-
(2008) Trends in Molecular Medicine. 14, 5, p. 199-209 Abstract
The development of metastasis requires the movement and invasion of cancer cells from the primary tumor into the surrounding tissue. To acquire such invasive abilities, epithelial cancer cells must undergo several phenotypic changes. Some of these, including alterations in cell adhesion and migration, are reminiscent of those observed during the developmental process termed epithelial-mesenchymal transition (EMT). Several master gene regulatory programs known to promote EMT during development have recently been discovered to play key roles in cancer progression. In particular, the regulation of cell adhesion molecules and the signaling pathways linking them to mechanisms of gene regulation has emerged as an important determinant of tumor cell invasion and metastasis. A deeper understanding of these mechanisms should allow both better diagnosis and the development of specific treatments for invasive cancer.
-
(2007) Journal of Cellular Biochemistry. 102, 4, p. 820-828 Abstract
A coordinated integration of cell-cell adhesion and the control of gene expression is essential for the development of multicellular, differentiated organisms. β-Catenin fulfils important regulatory functions in both cell-cell adhesion by linking cadherin adhesion receptors to the cytoskeleton, and also as a key element in the Wnt signaling pathway where it acts as cotranscriptional activator of target genes in the cell nucleus. Wnt signaling is involved in numerous aspects of embryonic development and in the control of tissue self-renewal in a variety of adult tissues. Hyperactivation of Wnt signaling, mostly by affecting β-catenin functions, is a hallmark of colon cancer and of many other human cancers. In this prospect, we discuss studies pointing to the molecular mechanisms that govern the integration between cell-cell adhesion and gene expression, as reflected in the switches between these two functions of γ-catenin in colon cancer cells.
-
-
(2007) Cancer Research. 67, 16, p. 7703-7712 Abstract
L1-CAM, a neuronal cell adhesion receptor, is also expressed in a variety of cancer cells. Recent studies identified L1-CAM as a target gene of β-catenin-T-cell factor (TCF) signaling expressed at the invasive front of human colon cancer tissue. We found that L1-CAM expression in colon cancer cells lacking L1-CAM confers metastatic capacity, and mice injected in their spleen with such cells form liver metastases. We identified ADAM10, a metalloproteinase that cleaves the L1-CAM extracellular domain, as a novel target gene of β-catenin-TCF signaling. ADAM10 overexpression in colon cancer cells displaying endogenous L1-CAM enhanced L1-CAM cleavage and induced liver metastasis, and ADAM10 also enhanced metastasis in colon cancer cells stably transfected with L1-CAM. DNA microarray analysis of genes induced by L1-CAM in colon cancer cells identified a cluster of genes also elevated in a large set of human colon carcinoma tissue samples. Expression of these genes in normal colon epithelium was low. These results indicate that there is a gene program induced by L1-CAM in colon cancer cells that is also present in colorectal cancer tissue and suggest that L1-CAM can serve as target for colon cancer therapy.
-
(2007) Cancer Research. 67, 14, p. 6844-6853 Abstract
Cancer cells become metastatic by acquiring a motile and invasive phenotype. This step requires remodeling of the actin cytoskeleton and the expression of exploratory, sensory organelles known as filopodia. Aberrant β-catenin-TCF target gene activation plays a major role in colorectal cancer development. We identified fascin1, a key component of filopodia, as a target of β-catenin-TCF signaling in colorectal cancer cells. Fascin1 mRNA and protein expression were increased in primary cancers in a stage-dependent manner. Fascin1 was exclusively localized at the invasive front of tumors also displaying nuclear β-catenin. Forced expression of fascin1 in colorectal cancer cells increased their migration and invasion in cell cultures and caused cell dissemination and metastasis in vivo, whereas suppression of fascin1 expression by small interfering RNA reduces cell invasion. Although expression of fascin1 in primary tumors correlated with the presence of metastases, fascin1 was not expressed in metastases. Our studies show that fascin1 expression is tightly regulated during development of colon cancer metastases and is a novel target of β-catenin-TCF signaling. We propose that transient up-regulation of fascin1 in colorectal cancer promotes the acquisition of migratory and invasive phenotypes that lead to metastasis. Moreover, the expression of fascin1 is down-regulated when tumor cells reach their metastatic destination where migration ceases and proliferation is enhanced. Although metastasis to vital organs is often the cause of mortality, only limited success has been attained in developing effective therapeutics against metastatic disease. We propose that genes involved in cell migration and invasion, such as fascin1, could serve as novel targets for metastasis prevention.
-
(2006) Molecular Cancer Therapeutics. 5, 11, p. 2861-2871 Abstract
The adenomatous polyposis coli or β-catenin genes are frequently mutated in colorectal cancer cells, resulting in oncogenic activation of β-catenin signaling. We tried to establish in vitro and in vivo models for selectively killing human cancer cells with an activated β-catenin/ T-cell factor (Tcf) pathway. We used a recombinant adenovirus that carries a lethal gene [p53-up-regulated modulator of apoptosis (PUMA)] under the control of a β-catenin/Tcf - responsive promoter (AdTOP-PUMA) to selectively target human colorectal cancer cells (SW480, HCT116, DLD-1, and LS174T), hepatocellular carcinoma (HepG2), and gastric cancer cells (AGS) in which the β-catenin/Tcf pathway is activated, and compared its efficiency in killing cancer cells in which this pathway is inactive or only weakly active. AdFOP-PUMA, carrying a mutant Tcf-binding site, was used as control virus. The combined effect of AdTOP-PUMA with several chemotherapeutic agents (5-florouracil, doxorubicin, and paclitaxel) was also evaluated. The effect of AdTOP-PUMA on colorectal cancer cells was also examined in nude mice: SW480 cells were infected with the AdTOP-PUMA and AdFOP-PUMA, and then inoculated s.c. into nude mice. The TOP-PUMA adenovirus inhibited cell growth in a dose-dependent fashion, depending on the signaling activity of β-catenin. The growth of cells displaying high levels of active β-catenin/Tcf signaling was inhibited after infection with AdTOP-PUMA, whereas that of cells with low levels of β-catenin signaling was not. Growth inhibition was associated with induction of apoptosis. Chemotherapy synergistically enhanced the effect of AdTOP-PUMA. A combination of the adenovirus system with standard therapy may improve the efficacy and reduce the toxicity of therapy in humans.
-
(2006) Journal of Biological Chemistry. 281, 5, p. 2901-2910 Abstract
Using a dual pipette assay that measures the force required to separate adherent cell doublets, we have quantitatively compared intercellular adhesiveness mediated by Type I (E- or N-cadherin) or Type II (cadherin-7 or -11) cadherins. At similar cadherin expression levels, cells expressing Type I cadherins adhered much more rapidly and strongly than cells expressing Type II cadherins. Using chimeric cadherins, we found that the extracellular domain exerts by far the dominant effect on cell adhesivity, that of E-cadherin conferring high adhesivity, and that of cadherin-7 conferring low adhesivity. Type I cadherins were incorporated to a greater extent into detergent-insoluble cytoskeletal complexes, and their cytoplasmic tails were much more effective in disrupting strong adherent junctions, suggesting that Type II cadherins form less stable complexes with β-catenin. The present study demonstrates compellingly, for the first time, that cadherins are dramatically different in their ability to promote intercellular adhesiveness, a finding that has profound implications for the regulation of tissue morphogenesis.
-
(2005) Cancer Research. 65, 24, p. 11605-11612 Abstract
Nr-CAM, a cell-cell adhesion molecule of the immunoglobulin-like cell adhesion molecule family, known for its function in neuronal outgrowth and guidance, was recently identified as a target gene of β-catenin signaling in human melanoma and colon carcinoma cells and tissue. Retrovirally mediated transduction of Nr-CAM into fibroblasts induces cell motility and tumorigenesis. We investigated the mechanisms by which Nr-CAM can confer properties related to tumor cell behavior and found that Nr-CAM expression in NIH3T3 cells protects cells from apoptosis in the absence of serum by constitutively activating the extracellular signal-regulated kinase and AKT signaling pathways. We detected a metalloprotease-mediated shedding of Nr-CAM into the culture medium of cells transfected with Nr-CAM, and of endogenous Nr-CAM in B16 melanoma cells. Conditioned medium and purified Nr-CAM-Fc fusion protein both enhanced cell motility, proliferation, and extracellular signal-regulated kinase and AKT activation. Moreover, Nr-CAM was found in complex with α4β1 integrins in melanoma cells, indicating that it can mediate, in addition to homophilic cell-cell adhesion, heterophilic adhesion with extracellular matrix receptors. Suppression of Nr-CAM levels by small interfering RNA in B16 melanoma inhibited the adhesive and tumorigenic capacities of these cells. Stable expression of the Nr-CAM ectodomain in NIH3T3 cells conferred cell transformation and tumorigenesis in mice, suggesting that the metalloprotease-mediated shedding of Nr-CAM is a principal route for promoting oncogenesis by Nr-CAM.
-
(2005) Journal of Cell Biology. 168, 4, p. 633-642 Abstract
A berrant β-catenin-TCF target gene activation plays a key role in colorectal cancer, both in the initiation stage and during invasion and metastasis. We identified the neuronal cell adhesion molecule L1, as a target gene of β-catenin-TCF signaling in colorectal cancer cells. L1 expression was high in sparse cultures and coregulated with ADAM10, a metalloprotease involved in cleaving and shedding L1's extracellular domain. L1 expression conferred increased cell motility, growth in low serum, transformation and tumorigenesis, whereas its suppression in colon cancer cells decreased motility. L1 was exclusively localized in the invasive front of human colorectal tumors together with ADAM10. The transmembrane localization and shedding of L1 by metalloproteases could be useful for detection and as target for colon cancer therapy.
-
(2005) Rise and Fall of Epithelial Phenotype. Boston, MA: . p. 191-202 (true Molecular Biology Intelligence Unit). Abstract
The cadherin-catenin system of cell-cell adhesion molecules plays a key role in determining cellular and tissue morphogenesis. The normal function of this molecular complex is indispensable during various stages throughout development, not only for determining the proper adhesive interactions between neighboring cells, but also for transducing the signals elicited by the Wnt pathway, mostly by β-catenin. The dual role of β-catenin in the assembly of cell-cell adherens junctions and its role as a cotranscriptional activator of target genes in the Wnt pathway is often disrupted in cancer cells. In this perspective, we discuss the interplay between the adhesive and signaling roles of the cadherin-catenin system, its regulation by various mechanisms, with special emphasis on its role in the development of cancer.
-
(2005) Development. 132, 2, p. 267-277 Abstract
Wnt/β-catenin signaling pathway is involved in the maintenance of the progenitor cell population in the skin, intestine and other tissues, and its aberrant activation caused by stabilization of β-catenin contributes to tumorigenesis. In the mammary gland, constitutive activation of Wnt/β-catenin signaling in luminal secretory cells results in precocious lobuloalveolar differentiation and induces adenocarcinomas, whereas the impact of this signaling pathway on the function of the second major mammary epithelial cell lineage, the basal myoepithelial cells, has not been analyzed. We have used the keratin (K) 5 promoter to target the expression of stabilized N-terminally truncated β-catenin to the basal cell layer of mouse mammary epithelium. The transgenic mice presented an abnormal mammary phenotype: precocious lateral bud formation, increased proliferation and premature differentiation of luminal epithelium in pregnancy, persistent proliferation in lactation and accelerated involution. Precocious development in pregnancy was accompanied by increased Myc and cyclin D1 transcript levels, and a shift in p63 variant expression towards the ΔNp63 form. The expression of ECM-degrading proteinases and their inhibitors was altered in pregnancy and involution. Nulliparous transgenic females developed mammary hyperplasia that comprised undifferentiated basal (K5/14-positive, K8- and α-smooth muscle-actin-negative) cells. Multiparous mice, in addition, developed invasive basal-type carcinomas. Thus, activation of β-catenin signaling in basal mammary epithelial cells affects the entire process of mammary gland development and induces amplification of basal-type cells that lack lineage markers, presumably, a subpopulation of mammary progenitors able to give rise to tumors.
-
(2004) Oncogene. 23, 27, p. 4745-4753 Abstract
Novel N-cadherin expression has been linked to the invasive phenotype in bladder tumors yet a primary role for N-cadherin in invasion has not been defined in this model. To address this, N-cadherin was stably transfected into E-cadherin expressing bladder carcinoma cells. This resulted in an enhanced invasive capacity in in vitro assays that was blocked by incubation with an N-cadherin function-blocking antibody in a dose-dependent manner. Analysis of the signaling pathway(s) implicated in N-cadherin-mediated invasion in bladder carcinoma cell lines revealed no correlation between MAPK signaling and invasion, in the presence or absence of fibroblast growth factor 2. Also, while MAPK and p38 kinase inhibitors did not alter the invasive behavior of these cells, an increase in the phosphorylation of Akt at serine-473 was detected in N-cadherin transfectants, suggestive of N-cadherin-mediated Akt activation in bladder cell invasion. Incubation of N-cadherin transfectants with either PI3 kinase or Akt inhibitors resulted in a significant decrease in the invasive capacity of these cells. Exposure of cells to PP2, a src family kinase inhibitor, also decreased the invasive potential of N-cadherin transfectants and resulted in reduced phosphorylation of Akt. The involvement of Akt signaling in bladder cell invasion was also supported by the inhibition of bladder cell invasion by cells constitutively expressing an activated Akt kinase, using the PI3 kinase and Akt inhibitors and PP2. These results suggest that activation of PI3/AKT kinase following N-cadherin expression contributes to the increased invasive potential of bladder carcinoma cells.
-
(2004) Oncogene. 23, 25, p. 4444-4453 Abstract
β-Catenin, a structural component of cell-cell adhesions, is also a potent signaling molecule in the Wnt pathway activating target genes together with Lef/Tcf transcription factors. In colorectal and many other types of cancer, β-catenin is hyperactive owing to mutations in β-catenin, or in components regulating β-catenin degradation. Deregulated β-catenin can cause the activation of p53, a key tumor suppressor mutated in most cancers. Activated p53 can feed back and downregulate β-catenin. Here we investigated the mechanisms involved in downregulation of β-catenin by p53. We found that the p53-mediated reduction in β-catenin involves enhanced phosphorylation of β-catenin on key NH2-terminal serines and requires CK1 and GSK-3β activities, both being components of the β-catenin degradation machinery. Mutations in these NH2-terminal β-catenin serines blocked the ability of p53 to enhance the turnover of β-catenin. p53 also induced a shift in the distribution of the scaffold molecule Axin to a Triton X-100-soluble fraction, and led to depletion of β-catenin from this Triton-soluble fraction. The majority of Axin and phosphorylated β-catenin, however, colocalized in Triton X-100-insoluble punctate aggregates near the plasma membrane, and kinetics studies indicated that in the presence of p53 the movement of Axin into and out of the Triton X-100-insoluble fraction is accelerated. These results suggest that p53 induces a faster mobilization of Axin into the degradation complex thereby enhancing β-catenin turnover as part of a protective mechanism against the development of cancer.
-
(2004) Cellular Microbiology. Boquet P., Cossart P., Rappuoli R. & Normark S.(eds.). 2nd ed. Washington, DC: . p. 121-138 Abstract
This chapter addresses the complex molecular interrelationships between cell adhesion and the transduction of transmembrane signals that affect cell adhesion and fate. It is shown here that adhesion sites such as focal contacts and cell-cell adherens junctions contain multimolecular protein complexes that participate both in the physical assembly of adhesion sites and the associated cytoskeleton and in the transduction of long-range growth, differentiation, and survival signals. The network of molecular interactions of the different adhesions, their involvement in the interaction with the cytoskeleton, and their particular role in adhesion mediated signaling are discussed in this chapter. Cell-cell adhesion is also mediated by a multitude of transmembrane receptor molecules including immunoglobulin superfamily cell adhesion molecules (CAMs), selectins, and cadherins. The transmembrane domain of matrix adhesions consists of adhesion receptors, mainly different members of the integrin superfamily. As may be expected from the fact that these receptors can interact with different matrix molecules, this domain is also quite heterogeneous with respect to the integrin composition. The importance of tension for triggering adhesion-dependent signal transduction is supported by recent findings where external forces were directly applied to cell-extracellular matrix (ECM) adhesion sites by a microneedle, by stretching an elastic substrate, or by laser trapping of cell surface-attached beads covered with adhesion ligands. Definitive molecular mechanisms responsible for microtubule directing to focal adhesions are not clear, but the Rho effector, Diaphanous (Dia1), might be involved in this process based on its effects on microtubule dynamics.
-
(2003) Journal of Cell Biology. 163, 4, p. 847-857 Abstract
Transcriptional repression of E-cadherin, characteristic of epithelial to mesenchymal transition, is often found also during tumor cell invasion. At metastases, migratory fibroblasts sometimes revert to an epithelial phenotype, by a process involving regulation of the E-cadherin-β-catenin complex. We investigated the molecular basis of this regulation, using human colon cancer cells with aberrantly activated β-catenin signaling. Sparse cultures mimicked invasive tumor cells, displaying low levels of E-cadherin due to transcriptional repression of E-cadherin by Slug. Slug was induced by β-catenin signaling and, independently, by ERK. Dense cultures resembled a differentiated epithelium with high levels of E-cadherin and β-catenin in adherens junctions. In such cells, β-catenin signaling, ErbB-1/2 levels, and ERK activation were reduced and Slug was undetectable. Disruption of E-cadherin-mediated contacts resulted in nuclear localization and signaling by β-catenin, induction of Slug and inhibition of E-cadherin transcription, without changes in ErbB-1/2 and ERK activation. This autoregulation of E-cadherin by cell-cell adhesion involving Slug, β-catenin and ERK could be important in tumorigenesis.
-
(2003) Cancer Research. 63, 20, p. 6626-6634 Abstract
Overexpression of the oncoprotein SKI correlates with the progression of human melanoma in vivo. SKI is known to curtail the growth inhibitory activity of tumor growth factor β through the formation of repressive transcriptional complexes with Smad2 and Smad3 at the p21Waf-1 promoter. Here, we show that SKI also stimulates growth by activating the Wnt signaling pathway. From a yeast two-hybrid screen and immunoprecipitation studies, we identified the protein FHL2/DRAL as a novel SKI binding partner. FHL2, a LIM-only protein, binds β-catenin and can function as either a transcriptional repressor or activator of the Wnt signaling pathway. SKI enhanced the activation of FHL2 and/or β-catenin-regulated gene promoters in melanoma cells. Among the SKI targets were microphthalmia-associated transcription factor and Nr-CAM, two proteins associated with melanoma cell survival, growth, motility, and transformation. Transient overexpression of SKI and FHL2 in ski-/- melanocytes synergistically enhanced cell growth, and stable overexpression of SKI in a poorly clonogenic human melanoma cell line was sufficient to stimulate rapid proliferation, decreasing the number of cells in the G1 phase of the cell cycle, and dramatically increasing clonogenicity, colony size and motility. Taken together, these results suggest that by targeting members of the tumor growth factor β and β-catenin pathways, SKI regulates crucial events required for melanoma growth, survival, and invasion.
-
(2003) Molecular Biology of the Cell. 14, 2, p. 585-599 Abstract
The Wnt/β-catenin/Tcf and IκB/NF-κB cascades are independent pathways involved in cell cycle control, cellular differentiation, and inflammation. Constitutive Wnt/β-catenin signaling occurs in certain cancers from mutation of components of the pathway and from activating growth factor receptors, including RON and MET. The resulting accumulation of cytoplasmic and nuclear β-catenin interacts with the Tcf/LEF transcription factors to induce target genes. The IκB kinase complex (IKK) that phosphorylates IκB contains IKKα, IKKβ, and IKKγ. Here we show that the cyclin D1 gene functions as a point of convergence between the Wnt/β-catenin and IκB pathways in mitogenic signaling. Mitogenic induction of G1-S phase progression and cyclin D1 expression was PI3K dependent, and cyclin D1-/- cells showed reduced PI3K-dependent S-phase entry. PI3K-dependent induction of cyclin D1 was blocked by inhibitors of PI3K/Akt/IκB/IKKα or β-catenin signaling. A single Tcf site in the cyclin D1 promoter was required for induction by PI3K or IKKα. In IKKα-/- cells, mitogen-induced DNA synthesis, and expression of Tcf-responsive genes was reduced. Reintroduction of IKKα restored normal mitogen induction of cyclin D1 through a Tcf site. In IKKα-/- cells, β-catenin phosphorylation was decreased and purified IKKα was sufficient for phosphorylation of β-catenin through its N-terminus in vitro. Because IKKα but not IKKα induced cyclin D1 expression through Tcf activity, these studies indicate that the relative levels of IKKα and IKKβ may alter their substrate and signaling specificities to regulate mitogen-induced DNA synthesis through distinct mechanisms.
-
(2002) Annals of the New York Academy of Sciences. 973, 1, p. 374-383 Abstract
The p53 tumor suppressor protein provides a major anti-cancer defense mechanism, as underscored by the fact that the p53 gene is the most frequent target for genetic alterations in human cancer. Recent work has led to the realization that p53 lies at the hub of a very complex network of signaling pathways that integrate a variety of intracellular and extracellular inputs. Part of this network consists of an array of autoregulatory feedback loops, where p53 exhibits very intricate interactions with other proteins known to play important roles in the determination of cell fate. We discuss two such loops, one involving the ?-catenin protein and the other centering on the Akt/PKB protein kinase. In both cases, the central module is the interplay between p53 and the Mdm2 protein, which inactivates p53 and targets it for rapid proteolysis. Whereas deregulated ?-catenin can lead to Mdm2 inactivation and p53 accumulation, active p53 can promote the degradation and down-regulation of ?-catenin. Similarly, Akt can block p53 activation by potentiating Mdm2, whereas activated p53 can tune down Akt in several different ways. In each case, the actual output of the loop is determined by the delicate balance between the opposing effects of its different components. Often, this balance is dictated by additional signaling processes that occur simultaneously within the same cell. Genetic alterations characteristic of cancer are capable of severely distorting this balance, thereby overriding the tumor suppressor effects of p53 in a manner that facilitates neoplastic conversion.
-
(2002) Cancer Research. 62, 20, p. 5947-5954 Abstract
β-Catenin and its close homologue plakoglobin (τ-catenin) are major constituents of submembranal cell-cell adhesion sites. In addition, β-catenin is a key component in the canonical Wnt pathway. Aberrantly activated β-catenin signaling contributes to cancer progression by inducing [in complex with lymphocyte enhancer factor (LEF)/T-cell factor (TCF)] the transcription of proliferation-related genes such as cyclin DI and c-myc. Plakoglobin can also activate LEF/TCF-mediated transcription. Excessive β-catenin signaling in MEF triggers a p53-mediated antiproliferative response by inducing the expression of ARF. We have demonstrated previously that plakoglobin also exerts a tumor-suppressive effect in certain cancer cell lines. To identify genes induced by β-catenin and plakoglobin, DNA microarray analysis was carried out, and PML was among those genes of which the expression was significantly elevated by both plakoglobin and β-catenin. Activation of the PML promoter by β-catenin and plakoglobin was LEF/TCF-independent. We found that PML forms a complex with β-catenin in cells, and the two proteins colocalize in the nucleus. In addition, PML, p300, and β-catenin cooperated in transactivation of a subset of β-catenin-responsive genes including ARF and Siamois but not cyclin D1. Retroviral expression of β-catenin, plakoglobin, or PML suppressed the tumorigenicity of p53-negative human renal carcinoma cells, thus pointing to a novel antioncogenic response triggered by catenins that is mediated by the induction of PML.
-
(2002) Genes & Development. 16, 16, p. 2058-2072 Abstract
β-catenin and plakoglobin (γ-catenin) are homologous molecules involved in cell adhesion, linking cadherin receptors to the cytoskeleton. β-catenin is also a key component of the Wnt pathway by being a coactivator of LEF/TCF transcription factors. To identify novel target genes induced by β-catenin and/or plakoglobin, DNA microarray analysis was carried out with RNA from cells overexpressing either protein. This analysis revealed that Nr-CAM is the gene most extensively induced by both catenins. Overexpression of either β-catenin or plakoglobin induced Nr-CAM in a variety of cell types and the LEF/TCF binding sites in the Nr-CAM promoter were required for its activation by catenins. Retroviral transduction of Nr-CAM into NIH3T3 cells stimulated cell growth, enhanced motility, induced transformation, and produced rapidly growing tumors in nude mice. Nr-CAM and LEF-1 expression was elevated in human colon cancer tissue and cell lines and in human malignant melanoma cell lines but not in melanocytes or normal colon tissue. Dominant negative LEF-1 decreased Nr-CAM expression and antibodies to Nr-CAM inhibited the motility of B16 melanoma cells. The results indicate that induction of Nr-CAM transcription by β-catenin or plakoglobin plays a role in melanoma and colon cancer tumorigenesis, probably by promoting cell growth and motility.
-
(2002) Journal of Cell Science. 115, 13, p. 2771-2780 Abstract
A novel phosphorylation-specific antibody (αpβ-catenin) was generated against a peptide corresponding to amino acids 33-45 of human β-catenin, which contained phosphorylated serines at positions 33 and 37. This antibody is specific to phosphorylated β-catenin and reacts neither with the non-phosphorylated protein nor with phosphorylated or non-phosphorylated plakoglobin. It weakly interacts with S33Y β-catenin but not with the S37A mutant. pβ-catenin is hardly detectable in normal cultured cells and accumulates (up to 55% of total β-catenin) upon overexpression of the protein or after blocking its degradation by the proteasome. Inhibition of both GSK-3β and the proteasome resulted in a rapid (t1/2=10 minutes) and reversible reduction in pβ-catenin levels, suggesting that the protein can undergo dephosphorylation in live cells, at a rate comparable to its phosphorylation by GSK-3β. pβ-catenin interacts with LEF-1, but fails to form a ternary complex with DNA, suggesting that it is transcriptionally inactive. Immunofluorescence microscopy indicated that pβ-catenin accumulates in the nuclei of MDCK and BCAP cells when overexpressed and is transiently associated with adherens junctions shortly after their formation. pβ-catenin only weakly interacts with co-transfected N-cadherin, although it forms a complex with the ubiquitin ligase component β-TrCP. SW480 colon cancer cells that express a truncated APC, at position 1338, contain high levels of pβ-catenin, whereas HT29 cells, expressing APC truncated at position 1555, accumulate non-phosphorylated β-catenin, suggesting that the 1338-1555 amino acid region of APC is involved in the differential regulation of the dephosphorylation and degradation of pβ-catenin.
-
-
(2001) EMBO Journal. 20, 17, p. 4912-4922 Abstract
Aberrant activation of β-catenin contributes to the onset of a variety of tumors. We report that a tumor-derived β-catenin mutant induces accumulation and activation of the p53 tumor suppressor protein. Induction is mediated through ARF, an alternative reading frame product of the INK4A tumor suppressor locus, in a manner partially dependent on the transcription factor E2F1. In wild-type mouse embryo fibroblasts, mutant β-catenin inhibits cell proliferation and imposes a senescence-like phenotype. This does not occur in cells lacking either ARF or p53, where deregulated β-catenin actually overrides density-dependent growth inhibition and cooperates with activated Ras in transformation. Thus, the oncogenic activity of deregulated β-catenin is curtailed by concurrent activation of the p53 pathway, thereby providing a protective mechanism against cancer. When the p53 pathway is impaired, deregulated β-catenin is free to manifest its oncogenic features. This can occur not only by p53 mutations, but also by ablation of ARF expression, as observed frequently in early stages of colorectal carcinogenesis.
-
(2001) American Journal of Pathology. 159, 1, p. 43-49 Abstract
Plakoglobin and its homologue β-catenin are cytoplasmic proteins that mediate adhesive functions by interacting with cadherin receptors and signaling activities by interacting with transcription factors. It has been suggested that plakoglobin can suppress tumorigenicity whereas β-catenin can act as an oncogene. We investigated the correlation between the expression pattern of N-cadherin, β-catenin, and plakoglobin and tumor behavior in primary tumors of 20 neuroblastoma patients of all stages and in 11 human neuroblastoma cell lines. N-cadherin and β-catenin were detected in 9 of 11 and 11 of 11 cell lines, respectively, whereas plakoglobin was undetectable or severely reduced in 6 of 11 cell lines. Tumor cells from 16 of 20 patients expressed N-cadherin and 20 of 20 patients expressed β-catenin at levels similar to those of normal ganglion cells. Plakoglobin was undetectable in 9 of 20 tumors. Plakoglobin deficiency in the primary tumors was significantly associated with adverse clinical outcome. Five of the patients with plakoglobin-negative tumors died whereas four patients are alive without evident disease. In contrast, all patients with plakoglobin-positive tumors are alive; 2 of 11 are alive with the disease and 9 of 11 are alive without evident disease. These results suggest that down-regulation of plakoglobin may be of prognostic value for neuroblastoma patients as predictor of poor outcome.
-
(2001) Molecular Microbiology. 41, 3, p. 561-573 Abstract
Group A streptococcus (GAS) induces its own entry into eukaryotic cells in vitro and in vivo. Fibronectin (Fn) bound to protein F1, a GAS surface protein, acts as a bridge connecting the bacterium to host cell integrins. This triggers clustering of integrins, which acquire a polar pattern of distribution similar to that of protein F1 on the GAS surface. A unique and transient adhesion complex is formed at the site of GAS entry, which does not contain α-actinin. Vinculin is recruited to the site of GAS entry but is not required for uptake. The invading GAS recruits focal adhesion kinase (FAK), which is required for uptake and is tyrosine phosphorylated. The Src kinases, Src, Yes and Fyn, enhance the efficiency of GAS uptake but are not absolutely required for GAS entry. In addition, Rac and Cdc42, but not Rho, are required for the entry process. We suggest a model in which integrin engagement by Fn-occupied protein F1 triggers two independent signalling pathways. One is initiated by FAK recruitment and tyrosine phosphorylation, whereas the other is initiated by the recruitment and activation of Rac. The two pathways subsequently converge to trigger actin rearrangement leading to bacterial uptake.
-
(2001) Molecular Biology of the Cell. 12, 4, p. 1177-1188 Abstract
Drosophila Armadillo and its mammalian homologue β-catenin are scaffolding proteins involved in the assembly of multiprotein complexes with diverse biological roles. They mediate adherens junction assembly, thus determining tissue architecture, and also transduce Wnt/Wingless intercellular signals, which regulate embryonic cell fates and, if inappropriately activated, contribute to tumorigenesis. To learn more about Armadillo/β-catenin's scaffolding function, we examined in detail its interaction with one of its protein targets, cadherin. We utilized two assay systems: the yeast two-hybrid system to study cadherin binding in the absence of Armadillo/β-catenin's other protein partners, and mammalian cells where interactions were assessed in their presence. We found that segments of the cadherin cytoplasmic tail as small as 23 amino acids bind Armadillo or β-catenin in yeast, whereas a slightly longer region is required for binding in mammalian cells. We used mutagenesis to identify critical amino acids required for cadherin interaction with Armadillo/β-catenin. Expression of such short cadherin sequences in mammalian cells did not affect adherens junctions but effectively inhibited β-catenin-mediated signaling. This suggests that the interaction between β-catenin and T cell factor family transcription factors is a sensitive target for disruption, making the use of analogues of these cadherin derivatives a potentially useful means to suppress tumor progression.
-
(2001) Molecular and Cellular Biology. 21, 20, p. 6768-6781 Abstract
β-Catenin is a cytoplasmic protein that participates in the assembly of cell-cell adherens junctions by binding cadherins to the actin cytoskeleton. In addition, it is a key component of the Wnt signaling pathway. Activation of this pathway triggers the accumulation of β-catenin in the nucleus, where it activates the transcription of target genes. Abnormal accumulation of β-catenin is characteristic of various types of cancer and is caused by mutations either in the adenomatous polyposis coli protein, which regulates β-catenin degradation, or in the β-catenin molecule itself. Aberrant accumulation of β-catenin in tumors is often associated with mutational inactivation of the p53 tumor suppressor. Here we show that overexpression of wild-type p53, by either transfection or DNA damage, down-regulates β-catenin in human and mouse cells. This effect was not obtained with transcriptionally inactive p53, including a common tumor-associated p53 mutant. The reduction in β-catenin level was accompanied by inhibition of its transactivation potential. The inhibitory effect of p53 on β-catenin is apparently mediated by the ubiquitin-proteasome system and requires an active glycogen synthase kinase 3β (GSK3β). Mutations in the N terminus of β-catenin which compromise its degradation by the proteasomes, overexpression of dominant-negative ΔF-β-TrCP, or inhibition of GSKβ activity all rendered β-catenin resistant to down-regulation by p53. These findings support the notion that there will be a selective pressure for the loss of wild-type p53 expression in cancers that are driven by excessive accumulation of β-catenin.
-
Recruitment of β-catenin to cadherin-mediated intercellular adhesions is involved in myogenic induction(2001) Journal of Cell Science. 114, 7, p. 1309-1319 Abstract
Cadherin-mediated cell adhesion is involved in muscle differentiation from early stages of myogenic induction to late stages of myoblast interaction and fusion. β-Catenin is a major constituent of cadherin-based adherens junctions and also serves as a signal transduction molecule that regulates gene expression during development. In this study, we explored the involvement of β-catenin in myogenic differentiation. We show here that shortly after a switch from growth to differentiation medium, β-catenin translocates to cell-cell junctions and its levels increase. We further show that elevation of β-catenin levels, induced either by inhibition of its breakdown, using LiCl, or by its overexpression, suppresses the formation of adherens junctions, resulting in a sharp decline in myogenin expression and an arrest of myogenic progression. Recruitment of β-catenin to adherens junctions after transfection with N-cadherin restores myogenin expression in the transfected cells. These results suggest that increased cadherin-mediated adhesion and translocation of β-catenin to adherens junctions are involved in activating the early steps of myogenic differentiation.
-
(2001) International Journal of Surgical Pathology. 9, 4, p. 273-279 Abstract
β-catenin is a cytoskeleton-associated signaling molecule shown to be elevated in various carcinomas but mostly in colon cancer owing to its impaired degradation. In contrast, its close homologue plakoglobin was shown to suppress the tumorigenicity of certain tumor cells. In the present study, we have used a semiquantitative immunohistochemical approach to evaluate the extent of nuclear localization of β-catenin in human colonic adenocarcinomas and adenomas and compared it to the distribution of plakoglobin in the same tissues. We show that β-catenin accumulates in the nuclei of the epithelium of primary and metastatic colonic adenocarcinoma as well as in colonic adenomas. In contrast, nuclear plakoglobin levels in these tissues were low, even compared to those found in epithelial cells of normal colon. These results support the view that the increase in β-catenin levels in colon cancer cells occurs early in the tumorigenic process, leading to its nuclear localization, not only in invasive adenocarcinoma, but also in colonic adenoma with mild dysplasia.
-
-
(2000) Journal of Biological Chemistry. 275, 42, p. 32649-32657 Abstract
The cyclin D1 gene encodes the regulatory subunit of a holoenzyme that phosphorylates and inactivates the pRB tumor suppressor protein. Cyclin D1 is overexpressed in 20-30% of human breast tumors and is induced both by oncogenes including those for Ras, Neu, and Src, and by the β-catenin/lymphoid enhancer factor (LEF)/T cell factor (TCF) pathway. The ankyrin repeat containing serine-threonine protein kinase, integrin-linked kinase (ILK), binds to the cytoplasmic domain of β1 and β3 integrin subunits and promotes anchorage-independent growth. We show here that ILK overexpression elevates cyclin D1 protein levels and directly induces the cyclin D1 gene in mammary epithelial cells. ILK activation of the cyclin D1 promoter was abolished by point mutation of a cAMP-responsive element-binding protein (CREB)/ATF-2 binding site at nucleotide -54 in the cyclin D1 promoter, and by overexpression of either glycogen synthase kinase-3β (GSK-3β) or dominant negative mutants of CREB or ATF-2. Inhibition of the PI 3-kinase and AKT/protein kinase B, but not of the p38, ERK, or JNK signaling pathways, reduced ILK induction of cyclin D1 expression. ILK induced CREB transactivation and CREB binding to the cyclin D1 promoter CRE. Wnt-1 overexpression in mammary epithelial cells induced cyclin D1 mRNA and targeted overexpression of Wnt-1 in the mammary gland of transgenic mice increased both ILK activity and cyclin D1 levels. We conclude that the cyclin D1 gene is regulated by the Wnt-1 and ILK signaling pathways and that ILK induction of cyclin D1 involves the CREB signaling pathway in mammary epithelial cells.
-
(2000) Journal of Biological Chemistry. 275, 30, p. 23368-23377 Abstract
Caveolin-1 is a principal component of caveolae membranes. In NIH 3T3 cells, caveolin-1 expression is dramatically up-regulated in confluent cells and localizes at areas of cell-cell contact. However, it remains unknown whether caveolin-1 is involved in cell-cell signaling. Here, we examine the potential role of caveolin-1 in regulating β-catenin signaling. β-Catenin plays a dual role in the cell, linking E-cadherin to the actin cytoskeleton and in Wnt signaling by forming a complex with members of the lymphoid enhancing factor (Lef-1) family of transcription factors. We show that E-cadherin, β-catenin, and γ-catenin (plakoglobin) are all concentrated in caveolae membranes. Moreover, we demonstrate that activation of β-catenin/Lef-1 signaling by Wnt-1 or by overexpression of β-catenin itself is inhibited by caveolin-1 expression. We also show that recombinant expression of caveolin-1 in caveolin-1 negative cells is sufficient to recruit β-catenin to caveolae membranes, thereby blocking β-catenin-mediated transactivation. These results suggest that caveolin-1 expression can modulate Wnt/β-catenin/Lef-1 signaling by regulating the intracellular localization of β-catenin.
-
(2000) Journal of Biological Chemistry. 275, 28, p. 21203-21209 Abstract
The cyclin D1 gene encodes the regulatory subunit of the holoenzyme that phosphorylates and inactivates the retinoblastoma pRB protein. Cyclin D1 protein levels are elevated by mitogenic and oncogenic signaling pathways, and antisense mRNA to cyclin D1 inhibits transformation by the ras, neu, and src oncogenes, thus linking cyclin D1 regulation to cellular transformation. Caveolins are the principal protein components of caveolae, vesicular plasma membrane invaginations that also function in signal transduction. We show here that caveolin-1 expression levels inversely correlate with cyclin D1 abundance levels in transformed cells. Expression of antisense caveolin-1 increased cyclin D1 levels, whereas caveolin-1 overexpression inhibited expression of the cyclin D1 gene. Cyclin D1 promoter activity was selectively repressed by caveolin-1, but not by caveolin-3, and this repression required the caveolin-1 N terminus. Maximal inhibition of the cyclin D1 gene promoter by caveolin-1 was dependent on the cyclin D1 promoter T-cell factor/lymphoid enhancer factor-1-binding site between -81 to -73. The T-cell factor/lymphoid enhancer factor sequence was sufficient for repression by caveolin-1. We suggest that transcriptional repression of the cyclin D1 gene may contribute to the inhibition of transformation by caveolin-1.
-
(2000) Molecular and Cellular Biology. 20, 12, p. 4238-4252 Abstract
β-Catenin and plakoglobin are highly homologous components of cell-cell adherens junctions linking cadherin receptors to the actin cytoskeleton. β- Catenin, in addition, activates transcription by forming a complex with LEF/TCF family transcription factors in the nucleus. Plakoglobin can also bind to LEF-1 and, when overexpressed in mammalian cells, enhances LEF-1- directed transcription. Plakoglobin overexpression, however, results in the elevation and nuclear translocation of endogenous β-catenin. We show here, by DNA mobility shift analysis, that the formation of a plakoglobin-LEF/TCF- DNA complex in vitro is very inefficient compared to a complex containing β- catenin-LEF-DNA. Moreover, in plakoglobin-transfected cells plakoglobin- LEF/TCF-DNA complexes were not formed; rather, the endogenous β-catenin, whose level is elevated by plakoglobin transfection, formed a β-catenin-LEF- DNA complex. Removal of the N- and C-terminal domains of both β-catenin and plakoglobin (leaving the armadillo repeat domain intact) induced plakoglobin- LEF-DNA complex formation and also enhanced β-catenin-LEF-DNA complexing, both with in vitro-translated components and in transfected cells. Transfection with these truncated catenins increased endogenous β-catenin levels, but the truncated catenins acted as dominant-negative inhibitors of β-catenin-driven transcription by forming transcriptionally inactive complexes with LEF-1. When these catenin mutants were prevented from entering the nucleus, by their fusion to the connexin transmembrane domain, they indirectly activated transcription by increasing endogenous β-catenin levels. These results suggest that overexpression of plakoglobin does not directly activate transcription and that formation of catenin-LEF-DNA complexes is negatively regulated by the catenin N- and C-terminal domains.
-
(2000) Oncogene. 19, 16, p. 1992-2001 Abstract
β-Catenin and plakoglobin are closely related armadillo family proteins with shared and distinct properties; Both are associated with cadherins in actin-containing adherens junctions. Plakoglobin is also found in desmosomes where it anchors intermediate filaments to the desmosomal plaques. β-Catenin, on the other hand, is a component of the Wnt signaling pathway, which is involved in embryonic morphogenesis and tumorigenesis. A key step in the regulation of this pathway involves modulation of β-catenin stability. A multiprotein complex, regulated by Wnt, directs the phosphorylation of β-catenin and its degradation by the ubiquitin-proteasome system. Plakoglobin can also associate with members of this complex, but inhibition of proteasomal degradation has little effect on its levels while dramatically increasing the levels of β-catenin. β-TrCP, an F-box protein of the SCF E3 ubiquitin ligase complex, was recently shown to play a role in the turnover of β-catenin. To elucidate the basis for the apparent differences in the turnover of β-catenin and plakoglobin we compared the handling of these two proteins by the ubiquitin-proteasome system. We show here that a deletion mutant of β-TrCP, lacking the F-box, can stabilize the endogenous β-catenin leading to its nuclear translocation and induction of β-catenin/LEF-1-directed transcription, without affecting the levels of plakoglobin. However, when plakoglobin was overexpressed, it readily associated with β-TrCP, efficiently competed with β-catenin for binding to β-TrCP and became polyubiquitinated. Fractionation studies revealed that about 85% of plakoglobin in 293 cells, is Triton X-100-insoluble compared to 50% of β-catenin. These results suggest that while both plakoglobin and β-catenin can comparably interact with β-TrCP and the ubiquitination system, the sequestration of plakoglobin by the membrane-cytoskeleton system renders it inaccessible to the proteolytic machinery and stabilizes it.
-
Molecular basis for cell adhesion and adhesion-mediated signaling(2000) Cellular Microbiology. Washington, DC: . p. 81-95 Abstract
This chapter addresses the complex molecular interrelationships between cell adhesion and the transduction of transmembrane signals that affect cell adhesion and fate. It is shown here that adhesion sites such as focal contacts and cell-cell adherens junctions contain multimolecular protein complexes that participate both in the physical assembly of adhesion sites and the associated cytoskeleton and in the transduction of long-range growth, differentiation, and survival signals. The network of molecular interactions of the different adhesions, their involvement in the interaction with the cytoskeleton, and their particular role in adhesion mediated signaling are discussed in this chapter. Cell-cell adhesion is also mediated by a multitude of transmembrane receptor molecules including immunoglobulin superfamily cell adhesion molecules (CAMs), selectins, and cadherins. The transmembrane domain of matrix adhesions consists of adhesion receptors, mainly different members of the integrin superfamily. As may be expected from the fact that these receptors can interact with different matrix molecules, this domain is also quite heterogeneous with respect to the integrin composition. The importance of tension for triggering adhesion-dependent signal transduction is supported by recent findings where external forces were directly applied to cell-extracellular matrix (ECM) adhesion sites by a microneedle, by stretching an elastic substrate, or by laser trapping of cell surface-attached beads covered with adhesion ligands. Definitive molecular mechanisms responsible for microtubule directing to focal adhesions are not clear, but the Rho effector, Diaphanous (Dia1), might be involved in this process based on its effects on microtubule dynamics.
-
(2000) Journal of Cell Science. 113, 18, p. 3127-3139 Abstract
β-Catenin can play different roles in the cell, including one as a structural protein at cell-cell adherens junctions and another as a transcriptional activator mediating Wnt signal transduction. Plakoglobin (γ-catenin), a close homolog of β-catenin, shares with β-catenin common protein partners and can fulfill some of the same functions. The complexing of catenins with various protein partners is regulated by phosphorylation and by intramolecular interactions. The competition between different catenin partners for binding to catenins mediates the cross-talk between cadherin-based adhesion, catenin-dependent transcription and Wnt signaling. Although plakoglobin differs from β-catenin in its functions and is unable to compensate for defects in Wnt signaling resulting from lack of β-catenin, recent evidence suggests that plakoglobin plays a unique role in Wnt signaling that is different from that of β-catenin. The functional difference between catenins is reflected in their differential involvement in embryonic development and cancer progression.
-
(1999) Journal of Cellular Biochemistry. 76, 1, p. 1-12 Abstract
Monomeric (G) actin was shown to be involved in inhibiting its own synthesis by an autoregulatory mechanism that includes enhanced degradation of the actin mRNA [Bershadsky et al., 1995; Lyubimova et al., 1997]. We show that the 3'-untranslated region (3'-UTR) of β-actin mRNA, but not its 5'- untranslated region, is important for this regulation. The level of full- length β-actin mRNA in cells was reduced when actin filaments were depolymerized by treatment with latrunculin A and elevated when actin polymerization was induced by jasplakinolide. By contrast, the level of actin mRNA lacking the 3'-UTR remained unchanged when these drugs modulated the dynamics of actin assembly in the cell. Moreover, the transfection of cells with a construct encoding the autoregulation-deficient form of β-actin mRNA led to very high levels of actin expression corn pared with transfection with the control actin construct and was accompanied by characteristic changes in cell morphology and the structure of the actin cytoskeleton. These results suggest that the autoregulatory mechanism working via the 3'-UTR of actin mRNA is involved in controlling the maintenance of a defined pool of actin monomers that could be necessary for the proper organization of the microfilament system and the cytoskeleton-mediated signaling.
-
(1999) EMBO Journal. 18, 11, p. 3054-3063 Abstract
β-catenin is a multifunctional protein, acting both as a structural component of the cell adhesion machinery and as a transducer of extracellular signals. Deregulated β-catenin protein expression, due to mutations in the β-catenin gene itself or in its upstream regulator, the adenomatous polyposis coli (APC) gene, is prevalent in colorectal cancer and in several other tumor types, and attests to the potential oncogenic activity of this protein. Increased expression of β-catenin is an early event in colorectal carcinogenesis, and is usually followed by a later mutational inactivation of the p53 tumor suppressor. To examine whether these two key steps in carcinogenesis are interrelated, we studied the effect of excess β-catenin on p53. We report here that overexpression of β-catenin results in accumulation of p53, apparently through interference with its proteolytic degradation. This effect involves both Mdm2-dependent and -independent p53 degradation pathways, and is accompanied by augmented transcriptional activity of p53 in the affected cells. Increased p53 activity may provide a safeguard against oncogenic deregulation of β-catenin, and thus impose a pressure for mutational inactivation of p53 during the later stages of tumor progression.
-
(1999) Proceedings of the National Academy of Sciences of the United States of America. 96, 10, p. 5522-5527 Abstract
β-Catenin plays a dual role in the cell: one in linking the cytoplasmic side of cadherin-mediated cell-cell contacts to the actin cytoskeleton and an additional role in signaling that involves transactivation in complex with transcription factors of the lymphoid enhancing factor (LEF-1) family. Elevated β-catenin levels in colorectal cancer caused by mutations in β- catenin or by the adenomatous polyposis coli molecule, which regulates β- catenin degradation, result in the binding of β-catenin to LEF-1 and increased transcriptional activation of mostly unknown target genes. Here, we show that the cyclin D1 gene is a direct target for transactivation by the β-catenin/LEF-1 pathway through a LEF-1 binding site in the cyclin D1 promoter. Inhibitors of β-catenin activation, wild-type adenomatous polyposis coil, axin, and the cytoplasmic tail of cadherin suppressed cyclin D1 promoter activity in colon cancer cells. Cyclin D1 protein levels were induced by β-catenin overexpression and reduced in cells overexpressing the cadherin cytoplasmic domain. Increased β-catenin levels may thus promote neoplastic conversion by triggering cyclin D1 gene expression and, consequently, uncontrolled progression into the cell cycle.
-
(1999) Advances in Molecular and Cell Biology. North A. J., Garrod D. R., Bittar E. E. & Chidgey M. A. J.(eds.). Vol. 28. p. 135-163 Abstract
Publisher Summary This chapter describes the studies on adhesive junctions that are linked to the microfilament system, both at cellcell contact areas (adherens junctions) and cellextracellular matrix (ECM) adhesion sites (focal adhesions). In multicellular organisms, special mechanisms have evolved that allow adhesion of their constituent cells to either the ECM or to their neighbors. The adhesive sites of cells with the ECM and other cells (also known as \u201cjunctions\u201d) allow the assembly of individual cells into ordered multicellular tissues and organs. The adhesions of cells with their environment comprise three major domains. The adhesion receptors, consisting of transmembrane molecules that are linked to the internal cytoskeleton by a submembranous plaque consisting of a multimolecular complex that contains both structural and signaling molecules. This molecular connection between the outside and inside of the cell provides the potential for transmitting mechanical forces and is also involved in the bidirectional signaling from the outside to the inside of the cell and vice versa. Cell adhesion is now viewed as fulfilling not only a structural role but also having a role in the interpretation of the information in the DNA into the 3-D pattern of cells and tissues. The biochemical and molecular composition of these junctions and the regulation of their assembly is described with their potential role in regulating the tumorigenic ability of cells, in conjunction with mechanisms of adhesion-mediated signaling.
-
(1999) Anticancer Molecules: Structure, Function, And Design. 886, p. 37-47 Abstract
β-Catenin and plakoglobin are homologous proteins having a dual role in cell adhesion and in transactivation together with LEF/TCF transcription factors. Overexpression of plakoglobin suppresses tumorigenicity, whereas increased β-catenin levels are considered oncogenic. We compared the nuclear translocation and transactivation by β-catenin and plakoglobin. Overexpression of each protein resulted in nuclear translocation and formation of structures that also contained LEF-1 and vinculin with β-catenin, but not with plakoglobin. Transfection of LEF-1 translocated endogenous β-catenin, but not plakoglobin into the nucleus. Chimeras of the Gal4 DNA-binding domain and the transactivation domains of either plakoglobin or β-catenin were equally potent in transactivation, but induction of LEF-1-responsive transcription was higher with β-catenin. Overexpression of wt plakoglobin or mutant β-catenin lacking the transactivation domain induced nuclear accumulation of the enodogenous β-catenin and LEF-1-responsive transactivation. The nuclear localization and constitutive β-catenin-dependent transactivation in SW480 cancer cells were inhibited by overexpressing cadherin or α-catenin. Moreover, transfecting the cytoplasmic tail of cadherin inhibited transactivation, by competition with LEF-1 in the nucleus for β-catenin binding. The results indicate that (1) plakoglobin and β-catenin differ in nuclear translocation and complexing with LEF-1 and vinculin, (2) LEF-1-dependent transactivation is mainly driven by β-catenin, (3) cadherin and α-catenin can sequester β-catenin, inhibit its transcriptional activity, and antogonize its oncogenic action.
-
(1998) Proceedings of the National Academy of Sciences of the United States of America. 95, 26, p. 15339-15344 Abstract
We studied the effect of N-cadherin, and its free or membrane-anchored cytoplasmic domain, on the level and localization of β-catenin and on its ability to induce lymphocyte enhancer-binding factor 1 (LEF-1)-responsive transactivation. These cadherin derivatives formed complexes with β-catenin and protected it from degradation. N-cadherin directed β-catenin into adherens junctions, and the chimeric protein induced diffuse distribution of β-catenin along the membrane whereas the cytoplasmic domain of N-cadherin colocalized with β-catenin in the nucleus. Cotransfection of β-catenin and LEF-1 into Chinese hamster ovary cells induced transactivation of a LEF-1 reporter, which was blocked by the N-cadherin-derived molecules. Expression of N-cadherin and an interleukin 2 receptor/cadherin chimera in SW480 cells relocated β-catenin from the nucleus to the plasma membrane and reduced transactivation. The cytoplasmic tails of N- or E-cadherin colocalized with β-catenin in the nucleus, and suppressed the constitutive LEF-1-mediated transactivation, by blocking β-catenin-LEF-1 interaction. Moreover, the 72 C-terminal amino acids of N-cadherin stabilized β-catenin and reduced its transactivation potential. These results indicate that β-catenin binding to the cadherin cytoplasmic tail either in the membrane, or in the nucleus, can inhibit β-catenin degradation and efficiently block its transactivation capacity.
-
(1998) Current Opinion in Cell Biology. 10, 5, p. 629-639 Abstract
Plakoglobin and β-catenin are homologous proteins functioning in cell adhesion and transactivation. Their activities are controlled by three types of interactions: those with cadherins in adherens junctions, linking them to the actin cytoskeleton; interactions in the nucleus, where they bind to transcription factors and stimulate gene expression; interactions of free cytoplasmic β-catenin with axin and adenomatous polyposis coli (APC) protein which target it for degradation. Studies in the past year have demonstrated the complex interplay between these three types of interactions and the different behavior of β-catenin and plakoglobin in their involvement in morphogenesis and tumorigenesis strongly suggesting that catenins play key roles in adhesion-mediated signaling.
-
(1998) Journal of Cell Biology. 141, 6, p. 1433-1448 Abstract
β-Catenin and plakoglobin are homologous proteins that function in cell adhesion by linking cadherins to the cytoskeleton and in signaling by transactivation together with lymphoid-enhancing binding/T cell (LEF/TCF) transcription factors. Here we compared the nuclear translocation and transactivation abilities of β-catenin and plakoglobin in mammalian cells. Overexpression of each of the two proteins in MDCK cells resulted in nuclear translocation and formation of nuclear aggregates. The β-catenin-containing nuclear structures also contained LEF-1 and vinculin, while plakoglobin was inefficient in recruiting these molecules, suggesting that its interaction with LEF-1 and vinculin is significantly weaker. Moreover, transfection of LEF-1 translocated endogenous β-catchin, but not plakoglobin to the nucleus. Chimeras consisting of Gal4 DNA-binding domain and the transactivation domains of either plakoglobin or β-catenin were equally potent in transactivating a Gal4-responsive reporter, whereas activation of LEF-1- responsive transcription was significantly higher with β-catenin. Overexpression of wild-type plakoglobin or mutant β-catenin lacking the transactivation domain induced accumulation of the endogenous β-catenin in the nucleus and LEF-1-responsive transactivation. It is further shown that the constitutive β-catenin-dependent transactivation in SW480 colon carcinoma cells and its nuclear localization can be inhibited by overexpressing N-cadherin or α-catenin. The results indicate that (a) plakoglobin and β-catenin differ in their nuclear translocation and complexing with LEF-1 and vinculin; (b) LEF-1-dependent transactivation is preferentially driven by β-catenin; and (c) the cytoplasmic partners of β- catchin, cadherin and α-catenin, can sequester it to the cytoplasm and inhibit its transcriptional activity.
-
(1998) Dynamical Networks in Physics and Biology. Beysens D. A. & Forgacs G.(eds.). Germany: . p. 41-49 (true Centre de Physique des Houches). Abstract
Cell adhesion to neighboring cells and to the extracellular matrix (ECM) plays important roles in cell motility, growth, differentiation and survival (Ben-Zeev, 1997; Geiger et al., 1995; Gumbiner, 1996). The molecular interactions at cell adhesion sites include transmembrane integrin-type receptors that link cells to the ECM, and cadherin receptors at cell-cell contact sites that are associated with submembranal plaque proteins that bridge between the cytoskeleton and the adhesion receptors (Geiger et al., 1995). Major junctional plaque proteins are vinculin, α-actinin and the catenins. In addition, recent studies have amply indicated the localization in the submembranal plaque of a large number of regulatory molecules involved in signal transduction (Fig. 1, and Geiger et al., 1995; Gumbiner, 1996). The submembranal plaque area is now viewed not only as a structural link that mediates cell adhesion, but also as an important component in the control of signal transduction regulating cell behavior. Tumor cells are often characterized by altered adhesion, disorganized cytoskeletal assembly and impaired adhesion-mediated signaling (Ben-Zeev, 1985, 1992, 1997). Many cancer cells are \u201canchorage independent\u201d and less susceptible to cell density-dependent inhibition of growth. Our previous studies have demonstrated that both the organization and expression of junctional plaque proteins is modulated during growth activation, differentiation and transformation (Ben-Zeev, 1991).
-
Junctional Plaque Proteins and the Regulation of Tumorigenesis(1998) Cytoskeleton and G-proteins in the Regulation of Cancer. Kuzumaki N.(eds.). Vol. 37. p. 26-31 (trueHokkaido University Medical Library series). Abstract
-
Cytoskeletal Plaque Proteins: Their Role in the Regulation of Tumorigenesis(1998) G Proteins, Cytoskeleton and Cancer. Maruta H. & Kohama K.(eds.). Austin, Texas, USA: . p. 101-109 (trueMolecular Biology Intelligence Unit). Abstract
[Book Description] Building upon the pioneering work of Klaus Weber, who in the 1970s discovered a key to the malignant transformation of normal cells by tumor virus infection or mutations via the disruption of actin cables and subsequent induction of membrane ruffling occurring with oncogenes such as v-Ras and v-Src, the global contributors aim toward the quantum leap of creating an anticancer Holy Grail that would attenuate the malignant activity of oncogenic mutants. The two dozen papers are organized under the four major headings of: actin-cytoskeleton, Ras/Rho family GTPases, links between G proteins and actin, and new anticancer drugs (e.g. farnesyltransferase inhibitors, SCH51344, and azatyrosine).
-
(1997) Cell structure and signaling. Bittar E. E.(eds.). Vol. 24. p. 125-163 (trueAdvances in Molecular and Cell Biology). Abstract
Cell adhesion to the extracellular matrix (ECM) and to neighboring cells plays a central role in complex biological processes including motility, growth, differentiation, and cell survival. The loss of adhesion-responsive regulation of cell behavior is a characteristic property of many cancer cells. The recently discovered molecular components that constitute the cell adhesion receptors, their association with the cytoskeleton via junctional plaque proteins, and the recruitment to these sites of a large number of regulatory molecules (kinases, phosphatases, oncogene, and tumor suppressor gene products) provide new opportunities for research on the molecular basis of adhesion-mediated signaling. Such studies revealed a cooperation between the organization and expression of these adhesive molecules, and their function in signaling in conjunction with soluble cytokines and growth factors. This cooperation between physical adhesion and signaling, with signaling by soluble factors, provides the molecular basis for the coordination required for tissue function.
-
(1997) Journal of Cell Biology. 139, 5, p. 1325-1335 Abstract
β-Catenin and plakoglobin (γ-catenin) are closely related molecules of the armadillo family of proteins. They are localized at the submembrane plaques of cell-cell adherens junctions where they form independent complexes with classical cadherins and α-catenin to establish the link with the actin cytoskeleton. Plakoglobin is also found in a complex with desmosomal cadherins and is involved in anchoring intermediate filaments to desmosomal plaques. In addition to their role in junctional assembly, β-catenin has been shown to play an essential role in signal transduction by the Wnt pathway that results in its translocation into the nucleus. To study the relationship between plakoglobin expression and the level of β-catenin, and the localization of these proteins in the same cell, we employed two different tumor cell lines that express N-cadherin, and α- and β-catenin, but no plakoglobin or desmosomal components. Individual clones expressing various levels of plakoglobin were established by stable transfection. Plakoglobin overexpression resulted in a dose-dependent decrease in the level of β-catenin in each clone. Induction of plakoglobin expression increased the turnover of β-catenin without affecting RNA levels, suggesting posttranslational regulation of β-catenin. In plakoglobin overexpressing cells, both β-catenin and plakoglobin were localized at cell-cell junctions. Stable transfection of mutant plakoglobin molecules showed that deletion of the N-cadherin binding domain; but not the α-catenin binding domain, abolished β-catenin downregulation. Inhibition of the ubiquitin-proteasome pathway in plakoglobin overexpressing cells blocked the decrease in β- catenin levels and resulted in accumulation of both β-catenin and plakoglobin in the nucleus. These results suggest that (a) plakoglobin substitutes effectively with β-catenin for association with N-cadherin in adherens junctions, (b) extrajunctional β-catenin is rapidly degraded by the proteasome-ubiquitin system but, (c) excess β-catenin and plakoglobin translocate into the nucleus.
-
(1997) Journal of Cellular Biochemistry. 65, 4, p. 469-478 Abstract
Regulation of the assembly and expression of actin is of major importance in diverse cellular functions such as motility and adhesion and in defining cellular and tissue architecture. These biological processes are controlled by changing the balance between polymerized (F) and soluble (G) actin. Previous studies have indicated the existence of an autoregulatory pathway that links the state of assembly and expression of actin, resulting in the reduction of actin synthesis after actin filaments are depolymerized. We have employed the marine toxins swinholide A and latrunculin A, both disrupting the organization of the actin-cytoskeleton, to determine whether this autoregulatory response is activated by a decrease in the level of polymerized actin or by an increase in monomeric actin concentrations in the cell. We showed that in cells treated with swinholide A the level of filamentous actin is decreased, and using a reversible cross-linking reagent, we found that actin dimers are formed. Latrunculin A also disassembled actin filaments, but produced monomeric actin, followed by a reduction in actin and vinculin expression, while swinholide A treatment elevated the synthesis of these proteins. In cells treated with both latrunculin A and swinholide A, dimeric actin was formed, and actin and vinculin synthesis were higher than in control cells. These results suggest that the substrate that confers an autoregulated reduction in actin expression is monomeric actin, and when its level is decreased by dimeric actin formation, actin synthesis is increased.
-
(1997) EMBO Journal. 16, 10, p. 2717-2729 Abstract
Shigella flexneri is the causative agent of bacillary dysentery in humans. Shigella invasion of epithelial cells is characterized by cytoskeletal rearrangements and formation of cellular projections engulfing the bacterium in a macropinocytic process. We show here that vinculin, a protein involved in linking actin filaments to the plasma membrane, is a direct target of Shigella during cell invasion. IpaA, a Shigella protein secreted upon cell contact, rapidly associates with vinculin during bacterial invasion. Although defective for cell entry, an ipaA mutant is still able to induce foci of actin polymerization, but differs from wild-type Shigella in its ability to recruit vinculin and α-actinin. Presumably, IpaA-vinculin interaction initiates the formation of focal adhesion-like structures required for efficient invasion.
-
(1997) Current Opinion in Cell Biology. 9, 1, p. 99-108 Abstract
In the past year, significant progress has been made in the attempt to understand the molecular mechanisms underlying signaling that is induced by cell-cell and cell-extracellular-matrix adhesion and that involves the cytoskeleton. In particular, molecules of the cytoplasmic plaques of cell-cell junctions have been shown to complex with transcription factors and to translocate into the nucleus. In addition, such junctional plaque proteins have been shown to act as effective suppressors of tumorigenesis.
-
(1997) Journal of Cell Science. 110, 8, p. 965-974 Abstract
The co-expression of vimentin and keratin-type intermediate filaments in the same cell was often reported to correlate with increased invasiveness and a more aggressive tumorigenic phenotype. To address the possible physiological relevance of these observations, we transfected simple keratins (K8 and 18) either individually, or in combination, into a tumorigenic but non-metastatic pancreatic adenocarcinoma that expresses vimentin but no keratins. Expression of K8 resulted in the stabilization of endogenous K19 in these cells, and formation of keratin filaments containing K8 and K19. Transfection of K18 yielded unstable K18 protein, but K18 could be stabilized when K8 was co-expressed in the same cells. Clones expressing K18 alone, or together with K8, displayed a reduced ability to grow in soft agar and decreased motility when compared to control, or K8/19 expressing cells. Moreover, K18 expressing cells were dramatically inhibited in their ability to form tumors when injected into syngeneic animals. The extent of suppression in the tumorigenicity of these cells correlated with the level of K18 expressed by these cells. The results show that K18 expression in cells may result in the suppression of the motile and tumorigenic abilities of this adenocarcinoma.
-
(1996) Electrophoresis. 17, 11, p. 1752-1763 Abstract
The morphology and functions of cells and tissues are determined, in a large part, by mechanical forces generated at cell-cell and cell-extracellular matrix (ECM) contacts. At these sites, transmembrane adhesion receptors of the integrin and cadherin families are linked, via their cytoplasmic domain, to the cytoskeleton by submembranal plaque proteins such as vinculin, α-actinin and the cell-cell junctional plaque proteins α- and β-catenin and plakoglobin (or γ-catenin). Recent studies have implicated this link of structural molecules between the outside and inside of the cell in signal transduction. We have shown that the expression of junctional plaque proteins is modulated during growth stimulation and differentiation, and is dramatically reduced in certain tumor cells. To study the functional significance of these changes in expression, we have used recombinant DNA technologies to overexpress or suppress the levels of junctional plaque proteins. In addition, we eliminated the expression of vinculin in embryonal stem (ES) cells and in the embryonal carcinoma F9 line by gene disruption employing homologous recombination. The results have indicated that moderate overexpression of cell-ECM plaque proteins results in reduced cell motility. In contrast, suppression of their expression, by antisense transfection, led to enhanced motility and conferred anchorage independent growth and tumorigenicity, upon injection into nude mice. These findings suggest that submembranal plaque proteins can act as effective tumor suppressors. In agreement with this notion, we found in several tumor cell lines diminished levels of junctional plaque proteins. Restoration of their level to that found in normal cells resulted in tumor suppression after their injection into experimental animals. Here we demonstrate the usefulness of the application of two dimensional (2-D) gel electrophoresis in these studies.
-
(1996) Journal of Cell Biology. 133, 1, p. 199-209 Abstract
Plakoglobin is a major component of the submembranal plaque of adherens junctions and desmosomes in mammalian cells. It is closely related to the Drosophila segment polarity gene armadillo which has a role in the transduction of transmembrane signals that regulate cell fate. Like its close homologue β-catenin, plakoglobin can associate with the product of the tumor suppressor gene APC that is linked to human colon cancer. We have studied the effect of plakoglobin overexpression, and the cooperation between plakoglobin and N-cadherin, on the morphology and tumorigenic ability of cells either lacking, or expressing cadherin and α- and β-catenin. Overexpression of plakoglobin in SV40-transformed 3T3 (SVT2) cells suppressed the tumorigenicity of the cells in syngeneic mice. Transfection with N-cadherin conferred an epithelial phenotype on the cell culture, but had no significant effect on the tumorigenicity of the cells. Cotransfection of plakoglobin and N-cadherin into SVT2 cells, however, was considerably more effective in tumor suppression than plakoglobin overexpression alone. Finally, transfection of plakoglobin into a human renal carcinoma cell line that expresses neither cadherins nor plakoglobin, or α- and β-catenin, resulted in a dose-dependent suppression of tumor formation by these cells in nude mice. Plakoglobin, in these cells, did not exhibit junctional localization and was diffusely distributed in the cytoplasm, with a significant amount of the protein also localized in the nucleus. The results suggest that plakoglobin can efficiently suppress the tumorigenicity of cells in the presence of, or independently of the cadherin-catenin complex.
-
Cell Adhesion and Cancer: Cytoskeletal Plaque Proteins as Tumor Suppressors(1996) Control Mechanisms of Carcinogenesis. Oesch F. & Hengstler JG.(eds.). p. 314-328 Abstract
-
(1995) Proceedings of the National Academy of Sciences of the United States of America. 92, 20, p. 9161-9165 Abstract
Vinculin, a major constituent of focal adhesions and zonula adherens junctions, is thought to be involved in linking the microfilaments to areas of cell-substrate and cell-cell contacts. To test the role of vinculin in cell adhesion and motility, we used homologous recombination to generate F9 embryonal carcinoma and embryonic stem cell clones homozygous for a disrupted vinculin gene. When compared to wild-type cells, vinculin-mutant cells displayed a rounder morphology and a reduced ability to adhere and spread on plastic or fibronectin. Decreased adhesion of the mutant cells was associated with a reduction in lamellipodial extensions, as observed by time-lapse video microscopy. The locomotive activities of control F9 and the vinculin-null ceils were compared in two assays. Loss of vinculin resulted in a 2.4-fold increase in cell motility. These results demonstrate an important role for vinculin in determining cell shape, adhesion, surface protrusive activity, and cell locomotion.
-
(1995) Journal of Cell Science. 108, 6, p. 2253-2260 Abstract
The assembly of focal adhesions was investigated in F9 embryonal carcinoma cells in which the expression of vinculin was eliminated by a targeted disruption of the vinculin gene. Vinculin-deficient F9 cells were capable of adhering to fibronectin-coated surfaces, though they displayed a reduced spreading compared to the parental cells. Transmission electron microscopy as well as interference reflection microscopy of live cells showed that vinculin-null F9 cells formed focal adhesions that were indistinguishable from those of the control cells. Fluorescent labeling for actin, talin, α-actinin, paxillin and phosphotyrosinated components indicated that the organization of all these focal contact-associated components was essentially identical in the vinculin-containing and vinculin-null cells. However, quantitative, digitized microscopy indicated that the intensity of fluorescence labeling in focal adhesions for α-actinin, talin and paxillin was significantly higher in cells lacking vinculin. The results suggest that there are multiple molecular mechanisms for the formation of focal adhesions in the absence of vinculin.
-
(1995) Journal of Cell Science. 108, 3, p. 1183-1193 Abstract
Actin filaments are major determinants of cell shape, motility and adhesion, which control important biological processes including embryonic development and wound healing. These processes are associated with changes in actin assembly, which is regulated by controlling the balance between polymerized and non-polymerized actin. To maintain a significant pool of non-polymerized actin, mechanism(s) linking actin synthesis to its state of polymerization were proposed. We have studied this relationship between actin synthesis and organization by modulating actin assembly using different drugs. Unassembled actin was increased in 3T3 cells using either the Clostridium botulinum C2 toxin, which ADP-ribosylates actin, or by latrunculin A, a Red Sea sponge product, which binds monomeric actin. The synthesis of actin was dramatically reduced in these cells owing to a concomitant decrease in actin RNA level. Similar results were obtained with HeLa cells grown in both monolayer and in suspension, suggesting that cell shape changes associated with drug treatment are not the primary cause for the effect on actin synthesis. In contrast, the scrape-loading of 3T3 cells with phalloidin, a stabilizer of polymerized actin that increased the level of assembled actin, resulted in elevated actin synthesis and RNA content. The expression of vinculin, a major component of adhesion plaques and cell-cell junctions, which is involved in actin-membrane associations, was altered in parallel with that of actin in cells treated with these drugs. The decrease in actin RNA resulted from destabilization of actin mRNA in cells where unassembled actin level was elevated. This is suggested by the unchanged transcription of actin in isolated nuclei from drug-treated cells, and by demonstrating that actin mRNA was degraded faster in cells after C2 toxin treatment than in control cells. This feedback control mechanism is mainly confined to the cytoplasm, as it remained active in enucleated cells. The results suggest the existence of an autoregulatory pathway for the expression of actin and other microfilament-associated proteins which is linked to the state of actin polymerization in the cell.
-
(1995) Cytoskeleton. Bittar E. E.(eds.). Vol. 12. p. 143-163 (trueAdvances in Molecular and Cell Biology). Abstract
This chapter describes adherens type junctions (AJ) modulation through changes in the level of expression of junctional components and explains that this modulation has a dramatic effect not only on cell structure, but also on cell motility and the tumorigenic ability of cells. Tyrosine phosphorylation of AJ proteins has been proposed to be a major mechanism in the signal transduction based on studies showing abundant tyrosine phosphorylation-dephosphorylation activity in AJ of both normal and transformed cells. The assembly of AJ apparently proceeds through an initial binding of the transmembrane contact receptor to its extracellular ligand (an ECM protein sequence, or a homologous cell adhesion (CAM) receptor). To determine the role of changes in the expression of AJ proteins in cell function, 3T3 cells are transfected with a full-length chicken vinculin cDNA construct and clones expressing stably different levels of the transgene were isolated.
-
(1994) Journal of Cell Science. 107, 7, p. 1773-1782 Abstract
Alpha-Actinin is an abundant actin crosslinking protein, also localized at adherens type junctions. In adhesion plaques, alpha-actinin can link the actin filaments to integrin via vinculin and talin, or directly by binding to the cytoplasmic domain of beta(1)-integrin. The expression of alpha-actinin is rapidly elevated in growth-activated quiescent cells, and is reduced in SV40-transformed 3T3 cells and various differentiating cell types (reviewed by Gluck, U., Kwiatkowski, D. J. and Ben-Ze'ev, A. Proc. Nat. Acad. Sci. USA 90, 383-387, 1993). To study the effect of changes in alpha-actinin levels on cell behavior, alpha-actinin expression was elevated in 3T3 cells by transfection with a full-length human nonmuscle alpha-actinin cDNA. To suppress alpha-actinin levels, 3T3 cells were transfected with an antisense alpha-actinin cDNA construct. Cells overexpressing alpha-actinin by 40-60% displayed a significant reduction in cell motility, as demonstrated by their slower locomotion into an artificial wound, and by forming shorter phagokinetic tracks on colloidal gold-coated substrata. 3T3 cells in which the expression of alpha-actinin was reduced to 25-60% of control levels, after antisense alpha-actinin transfection, had an increased cell motility. Moreover, such alpha-actinin-deficient 3T3 cells formed tumors upon injection into nude mice. The results demonstrate that modulations in alpha-actinin expression can affect, in a major way, the motile and tumorigenic properties of cells, and support the view that decreased alpha-actinin expression could be a common regulatory pathway to malignant transformation of 3T3 cells.
-
Beta-tropomyosin and alpha-actin are phenotypic markers for human intestinal smooth muscle cells in vitro(1994) Molecular and Cellular Differentiation. 2, 1, p. 45-60 Abstract
-
-
(1994) Cell Mechanics and Cellular Engineering. p. 273-293 (trueCell Mechanics And Cellular Engineering). Abstract
Malignant transformation is accompanied by diverse cellular manifestations including alterations in cell growth rate, and major changes in cell structure affecting cell adhesion and shape, motile activity, and cytoskeletal organization. Morphological changes are perhaps among the most conspicuous features of the transformed phenotype in culture, characterized by rounded cell shape with poorly organized microfilament bundles (Weber et al 1974; Pollack et al 1975), and aberrant adhesions (Ben-Zeev 1985; Raz and Ben-Zeev 1987). These multiple phenotypic changes between normal and tumor cells are consistent with the multistep theory of tumorigenesis, implying defects in numerous genes, including genes for molecules mediating cell adhesion (Ben-Zeev 1992; Hedrick et al 1993; Tsukita et al 1993). Cell adhesion to neighboring cells and to the extracellular matrix is mediated by transmembrane receptors of the Cadherin and integrin families of receptors (Hynes, 1987; Takeichi 1991, Figures 1,2). These receptor molecules are associated with cytoskeletal plaque proteins in the cytoplasmic face of the membrane to form different cellular junctions (Burridge et al 1988; Tsukita et al 1993).
-
(1994) Advances in Experimental Medicine and Biology. Vol. 358. p. 147-157 (trueActin: Biophysics, Biochemistry, And Cell Biology). Abstract
Cell adhesion to neighboring cells and to the extracellular matrix (ECM) plays a major role in cell and tissue morphogenesis (Edelman, 1992; Takeichi, 1991; Hynes, 1992). These complex, adhesion-related cellular processes are mediated through transmembrane contact receptors of the cadherin and integrin families of receptors (Takeichi, 1991; Hynes, 1992). In the cytoplasmic domain, these receptors interact with cytoskeletal plaque proteins such as vinculin, talin and α-actinin which anchor the microfilament system to junctional areas in adherens type junctions (AJ) in adhesion plaques, and to α and β catenin and plakoglobin in cell-cell AJ (Burridge et al., 1988; Geiger and Ginsberg, 1991; Geiger et al., 1992). The cascade of molecular interactions which links the outside to the inside of the cell defines cell shape and motility, and also has a function in signal transduction which results in effects on cell growth, differentiation, and gene expression (Ben-Zeev, 1991; 1992; Schwartz, 1992; Haskill and Juliano, 1993). Signaling through adhesion plaques is suggested to occur through changes in tyrosine phosphorylation (Burridge et al., 1992; Volberg et al., 1992). Moreover, recent studies have demonstrated that the changes in tyrosine phosphorylation of a cytoplasmic adhesion plaque tyrosine kinase (p125FAK) is common to adhesion related signaling and to growth factor, cytokine and neuropeptide induced signaling (Zachary and Rozengurt, 1992), and that tyrosine phosphorylation of p125FAK is constitutively activated in oncogene-transformed cells (Guan and Shalloway, 1992). These results suggest a convergence, in adhesion plaques, of signals transduced by cytokines, oncogenes and adhesion.
-
(1993) Journal of Cell Biology. 122, 6, p. 1285-1294 Abstract
The expression of vinculin, a major component of adhesion plaques and cell-cell junctions, is markedly modulated in cells during growth activation, differentiation, motility and cell transformation. The stimulation of quiescent cells by serum factors and the culturing of cells on highly adhesive matrices induce vinculin gene expression, whereas the transformation of fibroblast and epithelial cells often results in decreased vinculin expression (reviewed in Rodríguez Fernández, J. L., B. Geiger, D. Salomon, I. Sabanay, M. Zöller, and A. Ben-Ze'ev. 1992. J. Cell Biol. 119:427). To study the effect of reduced vinculin expression on cell behavior, 3T3 cells were transfected with an antisense vinculin cDNA construct, and clones displaying decreased vinculin levels down to 10-30% of control levels were isolated. These cells showed a round phenotype with smaller and fewer vinculin-positive plaques localized mostly at the cell periphery. In addition, they displayed an increased motility compared to controls, manifested by a faster closure of "wounds" introduced into the monolayer, and by the formation of longer phagokinetic tracks. Moreover, the antisense transfectants acquired a higher cloning efficiency and produced larger colonies in soft agar than the parental counterparts. The results demonstrate that the regulation of vinculin expression in cells can affect, in a major way, cell shape and motility, and that decreased vinculin expression can induce cellular changes reminiscent of those found in transformed cells.
-
(1993) Proceedings of the National Academy of Sciences of the United States of America. 90, 2, p. 383-387 Abstract
Human cytoskeletal α-actinin cDNA was transfected into highly malignant simian virus 40-transformed BALB/c 3T3 (SVT2) cells that express 6-fold lower levels of α-actinin than nontransformed BALB/c 3T3 cells. SVT2 clones expressing various levels of α-actinin were isolated and their structure and tumorigenic properties were determined. Transfected SVT2 clones expressing α-actinin at levels found in nontumorigenic 3T3 cells displayed a flatter phenotype, a decreased ability to grow in suspension culture in soft agar, and a marked reduction in their ability to form tumors in syngeneic BALB/c mice and in athymic nude mice. Clones overexpressing α-actinin at the highest level (about 2-fold higher than 3T3 cells) were completely suppressed in their ability to form tumors in syngeneic BALB/c mice. The results suggest that á-actinin, an actin-crosslinking protein that is also localized in cell junctions, may have an effective suppressive ability on the transformed phenotype.
-
(1992) Journal of Cell Biology. 119, 2, p. 427-438 Abstract
Transfection of chicken vinculin cDNA into two tumor cell lines expressing diminished levels of the endogenous protein, brought about a drastic suppression of their tumorigenic ability. The SV-40-transformed Balb/c 3T3 line (SVT2) contains four times less vinculin than the parental 3T3 cells, and the rat adenocarcinoma BSp73ASML has no detectable vinculin. Restoration of vinculin in these cells, up to the levels found in 3T3 cells, resulted in an apparent increase in substrate adhesiveness, a decrease in the ability to grow in soft agar, and suppression of their capacity to develop tumors after injection into syngeneic hosts or nude mice. These results suggest that vinculin, a cytoplasmic component of cell-matrix and cell-cell adhesions, may have a major suppressive effect on the transformed phenotype.
-
(1992) Experimental Cell Research. 202, 2, p. 477-486 Abstract
The expression of the adherens junction proteins vinculin, α-actinin, and talin was compared in serum-stimulated 3T3 cells and in regenerating rat liver following partial hepatectomy. The levels of vinculin RNA and protein synthesis were rapidly and transiently elevated in growth-activated fibroblasts (peaking at 2-3 h) and in regenerating liver (at 4-8 h), proceeding the replicative stage. α-Actinin expression was also induced, but more slowly (peaking at 6-8 h in 3T3 cells and at 28 h in regenerating liver), and remained elevated when DNA synthesis was proceeding in both systems. The expression of talin RNA was only slightly elevated in 3T3 cells following serum stimulation, and it remained largely unchanged in regenerating liver. The levels of RNA coding for fibronectin and for the β1-integrin subunit were transiently and extensively induced during liver regeneration (fibronectin with a peak at 8 h and β1-integrin at 12 h). The uvomorulin RNA level, and the expression of the liver-specific genes albumin and transthyretm, decreased in regenerating liver. The results suggest a physiologically significant regulation in the expression of structural components which link the extracellular matrix to the microfilament system in growth-activated fibroblasts and in regenerating liver.
-
Cytoarchitecture and signal transduction(1992) Critical Reviews in Eukaryotic Gene Expression. 2, 3, p. 265-281 Abstract
-
(1992) Cell Motility and the Cytoskeleton. 22, 2, p. 127-134 Abstract
The content of vinculin, a cytoplasmic protein found in focal contacts and cellcell junctions, was increased in BALB/c 3T3 cells by gene transfection. The vinculin expressed from the full length chicken cDNA, incorporated into focal contacts and its pattern was identical to that of the endogenous protein. Cells stably expressing vinculin by 20% over the endogenous level had altered locomotory properties. In these cells, the ability to migrate into a wound formed in a confluent monolayer and the locomotion of individual cells were drastically reduced. The results provide direct evidence that cell locomotion can be regulated by modulating vinculin expression. © 1992 WileyLiss, Inc.
-
-
(1991) BioEssays. 13, 5, p. 207-212 Abstract
Cell shape and cell contacts are determined by transmembrane receptormediated associations of the cytoskeleton with specific extracellular matrix proteins and with ligands on the surface of adjacent cells. The cytoplasmic domains of these microfilamentmembrane associations at the adherens junction sites, also Iocalize a variety of regulatory molecules involved in signal transduction and gene regulation. The stimulation of cells with soluble polypeptide factors leads to rapid changes in cell shape and microfilament component organization. In addition, this stimulation also activates the phosphoinositide signaling pathway. Recently, a linkage between actinbinding proteins and the phosphoinositide signaling pathway, was discovered. It is Suggested that by the association with the second messenger system, and/or by controlling the localization of regulatory molecules, the cytoskeleton may regulate gene expression.
-
(1991) Ultrastructure of the Ovary. Boston, MA: . p. 101-112 (trueElectron Microscopy in Biology and Medicine). Abstract
Granulosa cells (GC) play an important physiological role in the function of the mammalian ovary. They nurse the oocyte through gap junctions established between the egg and the surrounding first layer of GC, the corona radiât a. The modulation of communication between the oocyte and these cells is an important parameter involved in controlling ovum maturation and ovulation [1, 2, 3]. Upon luteinization, GC serve as a main source of progesterone in the female and acquire characteristic anatomical and biochemical features for optimal production of this steroid hormone [2, 3, 4, 5] (Fig. 7-1).
-
(1990) Oncogene. 5, 11, p. 1707-1711 Abstract
The expression of p53, a transformation associated protein, has been found to be regulated during the cell cycle. We show here that the subcellular localization of p53 varies throughout the cell cycle. In growth stimulated Balb/c 3T3 cells, p53 is produced at elevated levels and the newly synthesized protein accumulates in the cytoplasm during the G1 phase. Around the beginning of the S phase, p53 accumulates in the cell nucleus, where it stays for about 3 h. Following this initial step of DNA synthesis, p53 is no longer found in the nuclear compartment, but rather accumulates in the cytoplasm. This modulation in the subcellular localization of p53 suggests that the protein is spatially regulated during cell cycle.
-
(1990) Developmental Biology. 142, 1, p. 115-128 Abstract
The expression of the different tropomyosin isoforms was analyzed in primary granulosa cell cultures and in established granulosa cell lines cotransfected with SV40 and Ha-ras DNA which retain a high steroidogenic response to cAMP stimulation. In contrast to normal cells which greatly reduce the expression of all tropomyosin isoforms during development of steroidogenic ability, in the doubly transformed cells only the synthesis of the high molecular weight isoforms nos 2 and 3 was decreased. The expression of isoforms 1 and 5 was elevated in the cotransfected lines and that of tropomyosin 1 was further enhanced by cAMP stimulation. The increased synthesis of tropomyosins 1 and 5 is unique to SV40 transformation, since it was observed also in cells transfected with SV40 DNA alone. These cells displayed a well organized microfilament system, but have lost the ability to differentiate. The reduced expression of tropomyosins 2 and 3 and a poorly organized microfilament system appear to be a dominant feature of both the highly differentiated normal- and transformed-granulosa cells. It is suggested that the switches in tropomyosin isoform expression during development of the steroidogenic phenotype and in cell transformation may account for necessary changes in microfilament organization which accompany these cellular processes.
-
(1990) Molecular Biology of the Cell. 1, 9, p. 621-636 Abstract
When stimulated with serum, quiescent Balb/C-3T3 fibroblasts were found to induce vinculin transcription transiently within 30 min, followed by accumulation of vinculin mRNA and protein synthesis between 2 and 4 h after stimulation and a decrease to the basal level by 6-8 h. Platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and 12-O-tetradecanoylphorbol-13-acetate (TPA) each could elicit a similar response, albeit to a lesser extent, whereas epidermal growth factor (EGF) was inefficient in inducing vinculin expression. In cells stimulated with serum and cycloheximide, vinculin expression was superinduced and vinculin mRNA levels persisted longer than in cells stimulated with serum alone. Cells arrested in the presence of serum by anchorage denial in methyl cellulose suspension culture also induced vinculin expression and formed large vinculin positive plaques when reattaching and spreading on the substrate in the presence of serum. Cells replated from suspension culture in the absence of serum on either plastic or extracellular matrix (ECM) components were capable of extensive spreading, but failed to elevate vinculin expression and displayed diffuse vinculin staining. The results indicate that the changes in vinculin organization and expression in response to growth factor stimulation may reflect either a necessary step in the progression through the cell cycle or a response related to complex cellular processes such as wound repair and embryogenesis.
-
Regulation of cell contacts, cell configuration, and cytoskeletal gene expression in differentiating systems(1990) Mechanisms of Differentiation. p. 143-173 (trueMechanisms of Differentiation: Modulation of Differentiation by Exogenenous Agents). Abstract
-
(1990) Electrophoresis. 11, 3, p. 191-200 Abstract
Twodimensional gel electrophoresis was used to study the regulation of cytoskeletal protein synthesis during growth activation and development of the differentiated phenotype. We demonstrated a correlation between the state of organization and the expression of the respective cytoskeletal protein by showing that depolymerization of microtubules leads to a rapid decrease in new tubulin synthesis. We found that the synthesis of vimentin in both fibroblasts and epithelial cells correlates with extensive cell spreading on the substrate, while cytokeratin synthesis is maximal when cell to cell contacts are abundant. The analysis of cytoskeletal elements, involved directly in the formation of cell contacts, revealed that the level of vinculin synthesis is dependent on the extent of adherent type of cell contacts formed. Moreover, we found that the transient disappearance of vinculin from adhesion plaques of quiescent fibroblasts in response to serum factors was followed by an induction of vinculin mRNA and protein synthesis. The morphological changes associated with establishment of the differentiated phenotype were also found to include changes in the expression of the cytoskeletalextracellular matrix complex. This was demonstrated in several differentiating systems: in 3T3 preadipocytes which change their shape from a fibroblastic to a spherical shape when stimulated to differentiate with adipogenic medium, we observed a decrease in mRNA levels and in the synthesis of fibronectin, βintegrin, and the microfilament proteins, vinculin, αactinin, tropomyosin and actin. The culturing of these cells on a certain extracellular matrix prevented the morphological changes occurring in the presence of adipogenic medium and blocked the shifts in cytoskeletal and differentiationrelated gene expression. Similar changes in the organization and expression of cytoskeletal proteins were identified during maturation of primary ovarian granulosa cell cultures, stimulated with gonadotropic hormones to form highly steroidogenic cells. The cell rounding and aggregation occurring during this process were associated with a decreased synthesis of vinculin, αactinin, actin and the nonmuscle tropomyosins. The physiological relevance of these changes was suggested by the observation that the level of tropomyosin mRNA was lower in follicles of animals at late stages of granulosa cell maturation when compared to earlier stages. The expression of tissuespecific and cytoskeletal proteins was also determined in primary cultures of liver hepatocytes, maintained under conditions either favorable for growth or for expression of liverspecific functions. When DNA synthesis was elevated, cytoskeletal protein synthesis was high and that of liverspecific proteins was low. In contrast, when the expression of liver specific genes was maintained at high level, cytoskeletal gene expression and DNA synthesis were inhibited, as in the adult liver hepatocytes. Taken together, these results suggest that: (i) there is a close correlation between the mode of organization and expression of cytoskeletal proteins, (ii) such changes induced by the environment in the extracellular and intracellular matrices are programmed events occurring during growth activation and differentiation.
-
(1990) Molecular and Cellular Biology. 10, 12, p. 6565-6577 Abstract
The basic carboxy terminus of p53 plays an important role in directing the protein into the nuclear compartment. The C terminus of the p53 molecule contains a cluster of several nuclear localization signals (NLSs) that mediate the migration of the protein into the cell nucleus. NLSI, the most active domain, is highly conserved in genetically diverged species and shares perfect homology with consensus NLS sequences found in other nuclear proteins. The other two NLSs, II and III, appear to be less effective and less conserved. Although nuclear localization is dictated primarily by the NLSs inherent in the primary amino acid sequence, the actual nuclear homing can be modified by interactions with other proteins expressed in the cell. Comparison between wild-type p53 and naturally occurring mutant p53 showed that both protein categories could migrate into the nucleus of rat primary embryonic fibroblasts by essentially similar mechanisms. Nuclear localization of both proteins was totally dependent on the existence of functional NLS domains. In COS cells, however, we found that NLS-deprived wild-type p53 molecules could migrate into the nucleus by complexing with another nuclear protein, simian virus 40 large-T antigen. Wild-type and mutant p53 proteins differentially complexed with viral or cellular proteins, which may significantly affect the ultimate compartmentalization of p53 in the cell; this finding suggests that the actual subcellular compartmentalization of proteins may differ in various cell type milieux and may largely be affected by the ability of these proteins to complex with other proteins expressed in the cell. Experiments designed to test the physiological significance of p53 subcellular localization indicated that nuclear localization of mutant p53 is essential for this protein to enhance the process of malignant transformation of partially transformed cells, suggesting that p53 functions within the cell nucleus.
-
(1989) Trends in Biochemical Sciences. 14, 9, p. 377-382 Abstract
Studies on the dynamic biochemical and morphological events occurring during steroidogenesis in granulosa cells suggest that the organization and expression of the actin-cytoskeleton may play a major role in the transduction of endocrine and paracrine steroidogenic signals, and in the coordination between the organelles involved in this process. Since steroid hormones are not stored intracellularly, regulation of their production is dependent mainly on the expression of genes coding for membrane-bound steroidogenic enzymes. Recently, the expression of oncogenes of the ras family was also implicated in the regulation of steroidogenesis.
-
(1989) Developmental Biology. 135, 1, p. 191-201 Abstract
Granulosa cell differentiation in vitro in response to gonadotropins is characterized by major changes in cell shape, cell aggregation, and the organization of microfilaments. These changes are associated with enhanced steroidogenesis in maturing granulosa-lutein cells. Since nonmuscle tropomyosin isoforms were implicated in stabilizing actin filaments, we studied the organization and expression of tropomyosin in differentiating primary cultures of rat granulosa cells and during ovarian folliculogenesis and luteinization. In unstimulated primary granulosa cell cultures tropomyosin was found mainly along stress fibers. In differentiating cells tropomyosin staining was diffuse with sometimes a subcortical organization. The changes in tropomyosin organization were accompanied by a pronounced decrease in the synthesis, translation in vitro, and mRNA levels of all the rat nonmuscle tropomyosin isoforms, with a greater reduction in the higher molecular weight isoforms than in the smaller isoforms. Similar results were obtained whether cells were stimulated to differentiate with gonadotropins, with cAMP, by culturing cells on an extracellular matrix, or by treatment with cytochalasin B. The effect of cytochalasin B was reversible; upon removal of the drug tropomyosin synthesis increased to near control levels, while that of proteins associated with luteinization decreased drastically. RNA isolated from ovaries with follicles at the preantral, preovulatory stage and from corpora lutea contained decreased tropomyosin mRNA levels during ovarian luteinization when the level of RNA for a key steroidogenic enzyme, cytochrome P-450 cholesterol side chain cleavage (P-450 scc), increased. The results suggest a physiological relevance for the low level of tropomyosin expression in the mechanisms which bring about the morphological and biochemical development and maturation of granulosa cells.
-
(1989) Endocrinology. 124, 5, p. 2584-2594 Abstract
Primary cultures of granulosa cells can be stimulated to produce large amounts of progesterone by gonadotropins. This stimulation is associated with significant changes in the expression of several major proteins, as revealed by twodimensional gel electrophoresis. These changes include a decrease in the synthesis of actin cytoskeleton proteins and an increase in the synthesis of a few abundant proteins, one of which is a mammalian heat shock protein, hsp90. Under culture conditions that have previously been shown to bring about the maturation of granulosa cells into progesterone-producing cells (i.e. treatment with gonadotropins or cAMP or by disrupting the actin cytoskeleton with cytochalasin), an increased synthesis of hsp90 could be demonstrated. Freshly isolated granulosa cells isolated from PMSG-treated animals synthesize hsp90 at a much higher level than cells isolated from diethylstilbestrol-treated rats. Kinetic studies have shown that granulosa cells isolated from diethylstilbestrol- or PMSG-treated rats synthesize high levels of hsp90 if maintained in culture in the presence of gonadotropins, but rapidly decrease hsp90 synthesis in the absence of gonadotropins and increase the synthesis of actin cytoskeleton proteins. Furthermore, in cells cultured for 48 h in the presence of cytochalasin-B followed by incubation for 24 h in the absence of the drug, the synthesis of hsp90 and several other proteins characteristic of mature granulosa cells decreased, while that of the actin cytoskeleton increased. In vitro translation assays and Northern blot analyses suggest that hsp90 synthesis in gonadotropin-stimulated cells may be regulated by mRNA translational efficiency. Taken together with recent findings in which hsp90 was identified in complex with cytoplasmic steroid receptors and the hormonal regulation of hsp90 content in target tissues, the results support the notion that hsp90 plays a role in the control of steroid hormone action. (Endocrinology124: 2584-2594, 1989)Issue Section: Arti
-
(1989) Endocrinology. 124, 2, p. 1033-1041 Abstract
The organization and expression of the major cytoskeletal protein systems and of several cytoskeleton-associated proteins were analyzed in human granulosa cells obtained from preovulatory follicles in the course of an in vitro fertilization program. The cells were cultured in medium containing 5% fetal calf serum in the absence or presence of gonadotropins. Within several hours after the addition of hCG to cultured human granulosa cells, the cells acquired a rounded and aggregated morphology with numerous thin and long processes. Cells cultured without the gonadotropin had a flat and extended morphology on the substrate, with a well developed stress fiber system and numerous large vinculin-containing adhesion plaques. The [35S]methionine-labeled protein pattern of hCGtreated cells obtained by two-dimensional isoelectric focusing and sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed a 7- to 10-fold decrease in the synthesis of vinculin and the nonmuscle tropomyosin isoforms, and a 2- to 4-fold decrease in the synthesis of α-actinin and actin, without a comparable change in α- and β-tubulin synthesis. In the absence of gonadotropins, human granulosa cells synthesized both the intermediate filament (IF) protein vimentin and cytokeratin-type IF proteins 8 and 18 and, to a lesser extent, cytokeratins 7 and 19. By immunofluorescence, both type of IF networks were detected in untreated cells, while hCG-treated cells synthesized and contained only vimentin-type IF networks. Thus, maintenance of the steroidogenic capacity in the presence of gonadotropins in human granulosa-luteal cells involves specific changes in cell shape and in both the organization and the expression of the microfilament and IF systems.
-
Structure-function relationships in the differentiating granulosa cell.(1989) Progress in Clinical and Biological Research. 296, p. 121-130 Abstract
-
Plasticity in Cytoskeleton Expression During Differentiation in Response to Hormonal and Matrix Components(1989) Highlights of modern biochemistry. Kotyk A., Skoda J., Koska V. & Paces V.(eds.). Utrecht, Netherlands: . Vol. 2. p. 983-991 Abstract
-
Coordinated Regulation of Morphological and Biochemical Differentiation in Granulosa Cells(1989) Follicular Development and the Ovulatory Response. Tsafriri A. & Dekel N.(eds.). Rome, Italy: . Vol. 23. p. 39-52 (trueSerono symposia review). Abstract
-
(1989) Differentiation. 42, 2, p. 65-74 Abstract
The differentiation of 3T3 preadipocytes into adipocytes is characterized by major changes in cell morphology from a fibroblastic to a rounded shape and by the induction of gene expression related to lipid metabolism. We have studied the synthesis and mRNA levels of proteins involved in the formation of cell-matrix contacts and in defining cell shape to determine the role and molecular basis of these morphological changes during adipose conversion. When confluent preadipocyte cultures were stimulated with adipogenic medium there was a gradual decrease in the expression of fibronectin, β-integrin, actin and in the microfilament-associated proteins vinculin, α-actinin and tropomyosin. The changes in extracellular matrix and cytoskeletal mRNA levels were apparent before the accumulation of glycerophosphate dehydrogenase (GPD) mRNA and continued during the massive increase in GPD mRNA level. The culturing of preadipocytes on an extracellular matrix deposited on the dish by corneal endothelial cells, or on substrata coated with polylysine, prevented the morphological changes, the decrease in the level of assembled acin, the accumulation of lipid and the shifts in the expression of integrin, cytoskeletal proteins and GPD. In cells cultured on malleable hydrated collagen gels, adipocyte differentiation proceeded at normal rates. The results suggest that the regulated expression of proteins involved in the formation of the transmembrane linkage between the extracellular matrix and the microfilaments are programmed regulatory events that affect cell adhesion and thereby cell shape during adipocyte differentiation.
-
(1988) Proceedings of the National Academy of Sciences of the United States of America. 85, 7, p. 2161-2165 Abstract
Freshly isolated adult rat hepatocytes exhibit a flat, extended morphology when cultured on dried rat tail collagen in the presence of growth factors; they actively synthesize DNA and express high levels of cytoskeletal mRNAs and proteins (actin, tubulin, cytokeratins, vinculin, α-actinin, and desmoplakin), while exhibiting low levels of liver-specific mRNAs (albumin, α1-inhibitor III, and α1-antitrypsin) and limited synthesis and secretion of albumin. Hepatocytes cultured on hydrated gel matrix from the Engelbreth-Holm-Swarm (EHS) mouse tumor form small spherical aggregates and exhibit low DNA, cytoskeletal mRNA, and protein synthesis, while at the same time exhibiting elevated liver-specific mRNAs and albumin production; these cells, therefore, more nearly conform to the program of gene expression seen within the normal animal. Hepatocytes on hydrated rat tail collagen resemble those on dry collagen when cultured at low density, but at high density they form compact trabecular aggregates, synthesize negligible amounts of DNA, and maintain a pattern of gene expression resembling that of hepatocytes seeded on the EHS matrix. If cell morphology is compact, as on EHS or on hydrated rat tail collagen when densely populated, DNA synthesis and expression of cytoskeletal genes are low, while liver-specific mRNAs are abundant. When cells are extended the opposite is the case. Without the growth supplement DNA synthesis is low throughout but gene expression is little affected. These studies point to the importance of cell-cell and cell-matrix interactions in determining the differentiated phenotype of hepatocytes, and they reveal an inverse relationship between cytoskeletal and liver-specific protein expression.
-
(1988) International Journal of Cancer. 41, 2, p. 256-266 Abstract
The impact of interferongamma (IFN) treatment of tumor cells on nonadaptive and adaptive immune defense and its reflection by metastatic spread were evaluated using a weakly metastasizing variant of B16 melanoma (B16FI). Treatment of B16F1 with IFN resulted in a decrease in binding structures for NK cells and concomitantly in augmented metastasizing capacity. In line with this, activation of NK cells and Mo, which led to reduction of metastatic nodes, was less efficient with IFNtreated B16F1, while after elimination of nonadaptive immune defense, the number of metastases increased significantly, but irrespective of IFN treatment. On the other hand, IFNtreated B16F1 cells became more prone to killing by cytotoxic Tcells (CTL). This was due to increased lysability by CTL and to increased immunogenicity; i.e., a higher frequency of B16specific CTL was observed after immunization with IFNtreated than with untreated B16F1. The reverse phenomenon was observed with anomalous and/or lymphokineactivated killer cells (AK/LAK). The common cause of increased antigenicity and immunogenicity may reside in increased expression of classI and de novo expression of classII MHC antigens after IFN treatment. Increased antigenicity and immunogenicity of IFNtreated B16F1 was reflected by significant reduction of metastatic nodes, prolonged survival and increased TD100 in animals immunized with IFNtreated vs. untreated melanoma cells. Comparison of the divergent effects of IFN treatment on B16F1 melanoma cells showed that the benefit of increased antigenicity/immunogenicity clearly outweighed the disadvantage of reduced susceptibility to nonadaptive immune defense.
-
(1988) Developmental Biology. 129, 2, p. 505-515 Abstract
During the last stages of fetal life, the immature epithelial cells of the rat lung alveolus develop the properties of mature type 2 cells. Adult type 2 cells rapidly lose these same properties when isolated and maintained in cell culture. We have examined the synthesis of cytokeratin proteins by adult type 2 cells as they lose their differentiated characteristics during 1 week in culture, and of immature fetal alveolar epithelial cells as they differentiate either in utero or when cultured on an extracellular matrix. Freshly isolated adult type 2 cells synthesize four cytokeratins which by electrophoretic mobilities and Western blot analysis correspond to human cytokeratins Nos. 7, 8, 18, and 19. During 7 days in culture synthesis of cytokeratin No. 19 is dramatically decreased and cytokeratin No. 18 becomes the predominant acidic cytokeratin produced. Fetal lung epithelial cells at 18 days gestation lack most characteristics of mature type 2 cells. When freshly isolated, these cells synthesize cytokeratins Nos. 7, 8, and 18 but make only minimal amounts of cytokeratin No. 19. When these cells are allowed to mature either in utero or in culture on a whole basement membrane extract, they develop both the morphological characteristics and the pattern of cytokeratin synthesis of fully developed type 2 cells, with cytokeratins No. 19 being the major acidic cytokeratin produced.
-
Complex Formation Between the p53 Cellular Tumor Antigen and Nuclear Heat Shock Proteins(1988) Cellular and Molecular Biology of Tumors and Potential Application. Liss A. R., Minna J. & Kuehl WM.(eds.). Vol. 56. p. 247-259 (trueUCLA Symposia on Molecular & Cellular Biology). Abstract
-
(1988) Molecular Aspects of Papovaviruses. Aloni Y.(eds.). Boston, MA: . p. 239-268 (trueDevelopments in Molecular Virology). Abstract
Electrconmcroscopical and biochemical studies have shown an association between SV40 viral proteins, viral assembly, and the biogenesis of late SV40 RNA with the complex cellular networks of the nucleus and of the cytoplasm. Pulse-chase experiments revealed a rapid transport of viral capsid proteins from the cytoplasm to the nuclear matrix, where virus assembly occurs. The nuclear matrices of SV40-infected cells retain the majority of mature SV40 virions and the rapidly labeled SV40 nuclear RNA. Isolated nuclei from SV40-infected cells when incubated in the presence of [α-32P]UTP elongate the in vivo preinitiated SV40 RNA synthesizing both long viral RNA molecules and a 94 nucleotide long promoter-proximal viral RNA species (attenuator RNA). In contrast to rapidly labeled viral nuclear RNA, the attenuator RNA is not associated with the nuclear matrix. Pretreating the cells with proflavine that interferes with RNA secondary structure increases the amount of long viral RNA molecules which become associated with the nuclear matrix. Pretreatment with DRB (1-β-ribofuranosylbenzimidazole) which enhances premature termination of RNA synthesis enhances the accumulation of the attenuator RNA in the nuclei in a DNase and salt soluble fraction which is not associated with the matrix.
-
(1987) The journal of Biological chemistry. 262, 11, p. 5366-5376 Abstract
The link between the biochemical and morphological differentiation of granulosa cells was studied by investigating the organization and the expression of cytoskeletal proteins which determine cell shape and contacts. In cells treated with follicle-stimulating hormone (FSH), in a serum- and growth factor-free medium, or with other compounds which elevate cellular cAMP levels, the synthesis of the adherens junction proteins, vinculin, alpha-actinin, and actin was reduced significantly when compared to unstimulated cells (7-fold for vinculin, 5-fold for alpha-actinin, and 3-fold for actin). The in vitro translatability of the mRNAs coding for these proteins and the level of actin mRNA determined by RNA blot hybridization were generally reduced in differentiating cells. The synthesis and the organization of vimentin and tubulin was unaffected during this process, whereas the organization of actin and vinculin was dramatically affected, with FSH-treated cells displaying a diffuse pattern of actin and vinculin, with very little vinculin in adhesion plaques. Gonadotropin-releasing hormone agonist and the phorbol ester 12-O-tetradecanoylphorbol-13-acetate which are known to antagonize the cAMP-mediated biochemical differentiation of granulosa cells by reducing cAMP levels or by activating protein kinase C and phospholipid turnover, blocked to a large extent the FSH-induced effect on the adherens junction proteins. Epidermal growth factor, which blocked the FSH-induced cAMP increase, but not the FSH-induced progesterone production, failed to block the synthesis of vinculin, alpha-actinin, and actin. Cytochalasin B could induce steroidogenesis and similar changes in the synthesis of these cytoskeletal proteins, whereas fibronectin, which causes cell spreading, blocked in part the FSH-induced effect on the expression of cytoskeletal proteins. The modulation of cytoskeletal proteins may therefore be an essential feature of programmed differentiation events leading to the final phenotype of granulosa cells.
-
(1987) Cancer and Metastasis Reviews. 6, 1, p. 3-21 Abstract
The interaction of metastatic cells with the host environment occurs, to a large extent, through the cell surface, and the cell cytoskeletal system controls the distribution and motility of cell surface receptors. During metastasis, tumor cells migrate from one organ to another, and the dynamic properties and mechanochemical deformability of disseminated cells play a central role in the process. The studies described hereunder suggest an interrelationship between the cytoskeleton and cell adhesion, which can control and uagment the expression of the metastatic phenotype of neoplastic cells.
-
(1987) Journal of cell science. Supplement. 8, p. 293-312 Abstract
As a model for investigating gene regulation in relation to cell and tissue morphogenesis, we studied the expression of the adherens junction proteins, vinculin, α-actinin and actin, and that of desmosomal junctions containing the desmoplakincytokeratin complex, in response to changes in cell contacts and configuration. In monolayer or suspension cultures of kidney epithelial cells we found high levels of synthesis of cytokeratin and desmoplakin where extensive cellcell contacts were established. In contrast, cells in sparse monolayers had high levels of the vimentin-type intermediate filaments, but very low levels of cytokeratins and desmoplakin I. Whereas in kidney epithelial cells all cytokeratins were coordinately regulated in response to changes in culture conditions, in mammary epithelial cells a new 45×103 Mr. cytokeratin was induced in dense monolayer and suspension cultures. By treating cells with TPA, intercellular junctions were rapidly disrupted and expression of cytokeratin and desmoplakin was dramatically reduced; however, vimentin expression was not affected. In mammary epithelial cells only synthesis of the 45×103 Mr cytokeratin was reduced in TPA-treated cells. Thus the synthesis of the cytokeratindesmoplakin complex was coordinately regulated in response to changes in cellcell contact and cell shape in a way that is compatible with the organization of these cells in vivo.The relationship between the organization and expression of adherens junction proteins and their role in the acquisition of the differentiated phenotype was studied in fibroblasts and in differentiating ovarian granulosa cells. The synthesis of vinculin in cultured fibroblasts increased dramatically when the cell culture density was high, concomitant with the establishment of extensive cellsubstratum and cellcell contacts of the adherens type. When fibroblasts were plated on substrata of varying adhesiveness, to modulate cell shape from a flat and well-spread to a poorly adherent spherical shape, there was a relationship between vinculin organization and expression: vinculin synthesis decreased dramatically in round cells.The differentiation of freshly isolated ovarian granulosa cells (as measured by production of high levels of progesterone) in response to gonadotropic hormones was followed by dramatic changes in cell shape and organization and expression of adherens junction proteins. Cell shape changed from a flat fibroblastic type to a spherical one, with a reduction in vinculin-containing plaques and the disappearance of actin-containing stress fibres. Synthesis of vinculin, α-actinin and actin was significantly reduced but that of tubulin and vimentin was unchanged. Interestingly, when granulosa cells were plated on an extracellular matrix derived from endothelial cells, they underwent differentiation, even in the absence of gonadotropins, producing high levels of progesterone with similar changes in adherens junction protein synthesis and organization. The involvement of changes in organization and expression of adherens junctions in granulosa cell differentiation was further suggested by the observation that treatment with cytochalasin B alone was sufficient to induce simultaneous changes in adherens junction protein expression and progesterone production. Thus the modulation of expression and organization of these junctional proteins may be a central part of the programme of granulosa cell differentiation.The experimental systems described in this overview demonstrate a link between changes in cell contacts, cell configuration and the expression of differentiated tissue functions. They also provide us with a model with which to study the regulation of the organization and expression of junctional components in response to changes in cellular and tissue morphogenesis.
-
(1987) Differentiation. 34, 3, p. 222-235 Abstract
The gonadotropin-induced differentiation of granulosa cells in culture was studied, with particular attention being given to the organization and expression of cytoskeletal proteins involved in the formation of cell contacts, as well as to progesterone production. Gonadotropin-treated granulosa cells formed clusters of spherical cells containing few vinculin-containing focal contacts, exhibited a diffuse distribution of actin, and had few adherens junctions but more gap junctions than cells grown without the hormone. In gonadotropin-treated cells, the levels of synthesis of the cytoskeletal proteins, vinculin, α-actinin, and actin, were dramatically reduced, but the synthesis of the tubulins and vimentin was unaffected. Decreased levels of synthesis of these cytoskeletal proteins were also observed in an in vitro translation assay using poly (A)+ RNA from gonadotropin-treated cells. The hybridization of cytoplasmic RNA with cloned actin and vimentin cDNAs revealed a marked decrease in actin-RNA levels, but no change in vimentin-RNA levels in these cells. Such alterations in cytoskeletal-protein expression were also observed in cells treated with compounds that cause elevated cellular cAMP levels by acting at a stage beyond gonadotropin receptor stimulation. Furthermore, by keeping the cells in a spherical configuration in suspension culture, or by treating the cells with cytochalasin B, similar changes in the synthesis of these cytoskeletal proteins were observed. During this process, there was a concomitant increased in the production of progesterone (although to a much lesser extent in suspension culture) that occurred in parallel with the appearance of large mitochondria with lamellar-tubular cristae and a well-developed smooth endoplasmic reticulum, these features being characteristic of granulosa-lutein cells in vivo. Our results suggest that changes in cell shape and contact, together with the regulation of cytoskeletal elements that determine cellular morphogenesis, are part of the gonadotropin-controlled differentiation program in granulosa cells and may also occur during the maturation of these cells in vivo.
-
(1986) Trends in Biochemical Sciences. 11, 11, p. 478-481 Abstract
Cell contacts and morphogenesis are determined to a large extent by the organization of the intracellular cytoskeletal networks. Changes in cell shape and cytoplasmic organization are major regulators of growth, gene expression and cellular differentiation. The control of cytoskeletal gene expression by such morphological changes is by feedback control mechanisms which involved the state of organization of the respective cytoskeletal elements.
-
(1986) Experimental Cell Research. 166, 1, p. 47-62 Abstract
The analysis on two-dimensional isoelectric focusing and SDS polyacrylamide gels (2D gels) of the Triton X-100 and high salt-insoluble fraction of fibroblast cell lines, certain epithelial cell lines and granulosa cells revealed various amounts of a vimentin cleavage product, with a more basic pI and with a MW (1500-2000) lower than that of intact vimentin. This cleavage product of vimentin which constituted as much as 30% of the total vimentin in an established rat embryo fibroblast cell line (CREF), was detected by a monoclonal antivimentin antibody in whole cell and Triton-insoluble extracts, and it has a phosphorylated variant which can be degraded to form the "staircase pattern" on 2D gels similarly to intact vimentin. This processing of vimentin occurred mainly in dense cell cultures and it could not be induced in sparse cell cultures by inhibiting DNA synthesis with ara C, or by arresting cell growth in medium containing 0.1% serum. Transformation of CREF cells with intact wild-type (H5wt) and host-range cold-sensitive mutants (H5hr1 or H5d1101) of type 5 adenovirus (Ad5), or transformation of CREF cells by Ca2+-mediated DNA transfection with the transforming E1a (0-4.5 map units) or E1a + E1b (0-11.5 map units) region of Ad5 inhibits the cleavage of vimentin in dense cultures only at temperatures which are permissive for expression of the transformed phenotype. The transformation of cells with bovine papilloma virus type 1, with T24 ras oncogene, or with RSV does not interfere with the cleavage of vimentin. The organization of the vimentin network in dense cultures, where the vimentin cleavage occurs, is very different from that of sparse untransformed and sparse or dense Ad5-transformed cells. The possibility that the acidic amino acid-rich C-terminus of vimentin is cleaved in dense cell cultures in conjunction with the reorganization of the vimentin network and the inhibition of this cleavage by transformation with Ad5, are discussed.
-
(1986) Experimental Cell Research. 164, 2, p. 335-352 Abstract
The organization and synthesis of proteins involved in the formation and stabilization of desmosome-type junctions was investigated in cultured epithelial cells treated with a tumor promoter (12-O-tetradecanoyl-phorbol-13-acetate (TPA)). In Madin-Darby bovine (MDBK) and canine (MDCK) kidney cell colonies, TPA induced a rapid disruption of desmosomes and marked alterations in cell morphology. Within 4-6 h after TPA treatment, cell shape changed from cuboidal to highly irregular, with some very long extensions that contained cytokeratin fibrils, and many flat lamellar protrusions which were devoid of cytokeratin fibrils. These morphological changes in both MDBK and MDCK cells were followed by a dramatic and coordinated inhibition in the synthesis of all cytokeratins, 14-24 h after the addition of TPA, but without a similar effect on the synthesis of vimentin, which is coexpressed in these cells. In contrast, in dense cultures of MDBK and MDCK cells the synthesis of cytokeratins and the organization of desmosomal contacts were not affected by TPA. In an epithelial cell line derived from the bovine mammary gland (BMGE-H) the synthesis of an acidic cytokeratin of 45 kD, which was previously shown to be synthesized at high levels only in dense cultures, was dramatically inhibited by TPA treatment. Cell-free in vitro translation assays with mRNA from control and TPA-treated cells also demonstrated a decrease in the synthesis of cytokeratins in response to TPA. The inhibition of cytokeratin synthesis after TPA treatment was paralleled by a decrease in the synthesis of a high molecular weight (HMW) desmoplakin protein, which was abundant in dense MDBK and BMGE-H cells. The results with TPA-treated cells are suggestive of a coordinated down-regulation in the synthesis of only those cytokeratins and of a desmoplakin which were shown to be regulated by the extent of cell-cell contact. Cytokeratin phosphorylation in TPA-treated cells was low and reflected the decrease in their total mass, suggesting that it was not altered by TPA treatment. The possible linkage between the regulation of synthesis and organization of proteins involved in desmosome formation is discussed.
-
(1986) Cancer Research. 46, 2, p. 785-790 Abstract
The pattern of intermediate filament protein expression was studied in tumor cell variants of the BSp73 spontaneous rat adenocarcinoma of the pancreas exhibiting distinct morphology and metastatic phenotype. The non-metastasizing AS cells which adhere and spread on a solid substrate express only the vimentin type mesenchymal intermediate filament protein. The ASML metastatic cell variant which adheres but does not spread on the substrate expresses a complex pattern of cytokeratins characteristic of the adenocarcinoma of the pancreas and a low level of vimentin. The differences in the expression of the intermediate filament proteins between the variants were also reflected at the level of the corresponding mRNAs as revealed by RNA blot analysis with complementary DNA clones specific to vimentin and the acidic as well as the basic cytokeratin subfamily. When the two cell variants were cultured for 72 h in suspension culture on nonadhesive substrata the AS cells responded with a marked reduction in their vimentin synthesis. The ASML variant cells which are characterized by a round configuration in both monolayer and suspension culture continue to synthesize the same intermediate filament proteins under both culture conditions. The relationships among environmental conditions that affect cell shape and contacts, the shifts in the expression of intermediate filaments, and the metastatic property of tumor cells are discussed.
-
Regulation of cytoskeletal protein synthesis in normal and cancer cells(1986) Cancer Reviews. Vol. 4, p. 91-116 Abstract
-
(1986) Nature. 320, 6058, p. 182-185 Abstract
The protein p53 is capable of participating in neoplastic transformation1-3 and can form specific complexes with the large-T antigen of simian virus 40 (SV40)4-6. This interaction probably results in the stabilization of p53 (refs 7, 8) and may contribute to SV40-mediated transformation9,10. Several non-SV40-trans-formed cells also exhibit a stabilized p53 which is present in elevated levels 11-13. Recently, this stabilization was shown to coincide with the ability to precipitate a polypeptide (p68) of relative molecular mass (M r) 68,000-70,000 by anti-p53 monoclonal antibodies13-15. We now report that this co-precipitation indeed represents a specific complex between the two proteins; the complex sediments on a sucrose gradient as a relatively broad peak of 10-14S and can be dissociated in vitro. Furthermore, p68 is the HSP70 heat shock protein cognate, found in elevated levels in a p53-overproducing cell line. On heat-shock treatment of such overproduces, p53 also forms a complex with the related highly inducible HSP68.
-
(1986) Nature. 319, 6056, p. 787-791 Abstract
Recent studies have demonstrated the fundamental role of cell-substrate contacts and changes in cell shape in the regulation of cell growth, motility and differentiation1-7, but the molecular basis for these phenomena is poorly understood. Because of the involvement of cytoskeletal networks in cell morphogenesis and contact formation, it is of interest that the expression of genes encoding several cytoskeletal proteins is markedly affected by changes in cell contacts and configuration6-10. Because most of these phenomena involve changes in the form, extent or topology of cell contacts, we sought to determine whether the expression of components directly involved in the formation of cell-cell or cell-substrate contacts is affected by the respective cellular interactions. A suitable candidate for such analysis is vinculin, a cytoskeletal protein of relative molecular mass (Mr) 130,000 (130K), which is localized in focal contacts11-13 and intercellular adherens junctions14. The assembly of vinculin into a membrane-bound junctional plaque seems to be one of the earliest cellular responses to contact with exogenous substrates, leading to the subsequent local assembly of the actin-rich microfilament bundles15,16. Here we report on the regulation of vinculin synthesis in response to environmental conditions that affect cell shape and contacts.
-
(1986) Experimental Cell Research. 162, 1, p. 127-141 Abstract
The pattern of cell substrate interaction, the cell surface composition and the organization of cytoskeletal elements was studied in tumour cell variants of the BSp73 rat adenocarcinoma displaying different metastatic capabilities and cell configuration. The non-metastasizing AS variant cells adhered to the substrate and spread via vinculin-containing focal contacts. These cells also synthesized, secreted and assembled fibronectin at the pericellular area. The metastasizing ASML variant cells adhered to the substrate at a slower rate via thick cytoplasmic protrusions, but were removed from the substrate by trypsin-EDTA slower than the non-metastasizing AS variant cells. The ASML cells also synthesized very low levels of both vinculin and fibronectin, displayed a diffuse pattern of actin and tubulin organization, and were unable to spread on the substrate. Spreading could not be induced in the ASML cells by seeding the cells on an extracellular matrix derived from bovine corneal endothelial cells or on concanavalin A (conA)-coated substrates, or by the addition of db-cAMP to the medium. The metastasizing cells expressed a unique and abundant cell surface glycoprotein of Mr 170 000 which was also shedded into the growth medium. The relationships among the adhesive properties, the organization of cell surface components and of the cytoskeleton in the tumour cell variants, and the expression of their metastatic phenotype is discussed.
-
(1986) Proceedings of the National Academy of Sciences of the United States of America. 83, 9, p. 2894-2898 Abstract
The organization and the expression of cytoskeletal proteins involved in determining cell contact and shape were analyzed in granulosa cells during their differentiation on extracellular matrix (ECM)-coated culture plates. Rat granulosa cells from preovulatory follicles displayed an epithelial shape on ECM and formed multilayered aggregates with numerous gap junctions between neighboring cells. These cells had few actin cables and often only a diffuse pattern of actin and a low amount of vinculin in very thin focal adhesion sites. In contrast, cells grown on plastic formed a monolayer of flat cells with a reduced number of gap junctions but with numerous stress fibers and abundant large vinculin-containing focal contacts. On ECM, the cells were stimulated to produce high levels of progesterone, while only trace amounts of the steroid accumulated in cells on plastic dishes. Two-dimensional gel electrophoretic analysis of [35S]methionine-labeled cells revealed a dramatic decrease in vinculin, α-actinin, and actin synthesis in cells grown on ECM, as compared to cells grown on plastic, while the synthesis of the tubulins and of the intermediate filament protein vimentin remained unchanged. RNA blot analysis showed a marked decrease in actin mRNA levels in cells from ECM plates, while the level of tubulin mRNA remained essentially unchanged. It is concluded that the differentiation of granulosa cells on ECM in vitro is associated with changes in cell shape and cell contacts and that such changes in cell morphology are accompanied by simultaneous alterations in the organization and expression of cytoskeletal proteins that are involved in determining these cellular structures.
-
(1985) Annals of the New York Academy of Sciences. 455, 1, p. 597-613 Abstract
Keywords: Multidisciplinary Sciences
-
(1985) Cancer Research. 45, 6, p. 2632-2641 Abstract
The organization and synthesis of the vimentin-containing cytoskeletal network as well as the metastatic capability of B16-F1 melanoma cells were investigated in cells treated with cyclo-heximide (CH). The addition of CH to cells for 4-8 h resulted in a marked reversible alteration in the organization of the vimentin-containing network in B16-F1 melanoma cells as well as in a variety of epithelial and fibroblast cell lines. Treatment of cells with CH led to a reduction in the synthesis of vimentin, tubulin, and actin followed by a decrease in the concentrations of mRNAs coding for these proteins. However, out of these three cytoskeletal elements, only the organization of the intermediate filaments was disrupted by CH. Cells treated previously with CH and injected i.v. into syngeneic mice had diminished capacity to form lung metastases as compared to control untreated cells. This effect was reversible, and the metastatic capability recovered to the control level after the drug was removed from the growth medium for 16 h. The possibility that the organization and the synthesis of the cytoskeletal components are related and that the metastatic capability of B16 melanoma is influenced by the organization of the cytoskeletal networks are discussed.
-
Cell shape, the complex cellular networks, and gene expression. Cytoskeletal protein genes as a model system.(1985) Cell and muscle motility. Shay J. W.(eds.). US: . Vol. 6. p. 23-53 Abstract
-
(1985) Experimental Cell Research. 157, 2, p. 520-532 Abstract
The pattern of the intermediate type filament protein synthesis was examined in cultured bovine mammary gland epithelial (BMGE) cells under conditions of varied cell shape and cell-cell contact. In dense monolayer and suspension cultures BMGE cells expressed a new cytokeratin of 45 kD identified as a member of the acidic subfamily of cytokeratins. This polypeptide has a phosphorylated component and is dissociated from the cytokeratins complex in the presence of 6.5 M urea. The mRNA of the new cytokeratin accumulated in dense cell cultures, as revealed by in vitro translation in a cell-free system. In BMGE-H cells that express also vimentin, the synthesis of vimentin decreased dramatically in dense cell cultures, while the synthesis of the 45 kD cytokeratin was maximal under these conditions. The results suggest that the expression of certain cytokeratins and that of vimentin can be coordinately regulated by factors in the cellular environment that effect cell shape and cell surface contacts.
-
(1985) Biochimica et Biophysica Acta - Reviews on Cancer. 780, 3, p. 197-212 Abstract
Keywords: Biochemistry & Molecular Biology; Biophysics
-
The relationship between the organization and synthesis of vimentin and the metastatic capability of B16 melanoma cells(1985) Proceedings of the American Association for Cancer Research. 26, p. 187 Abstract
-
(1984) Experimental Cell Research. 153, 1, p. 99-108 Abstract
A nuclear matrix fraction was prepared from ovaries of the achiasmatic flour moth, Ephestia kuehniella, by removal of the chromatin, using detergent treatment of homogenized ovaries or dissected ovary tips followed by DNase digestion and high salt extraction. Removal of DNA and histones from the nuclei was demonstrated by Feulgen staining and polyacrylamide gel electrophoresis (PAGE), respectively. By light microscopy, ribbon-like structures similar in dimension to the synaptonemal complex were observed in the oocyte after digestion of the chromosomes. Electron microscopic examination of matrix preparations of pachytene cells showed a defined synaptonemal complex structure with both lateral and central elements. Such structures were not found in either the fully differentiated nurse cells or in follicle cells which were exposed to the same preparative technique concurrently. However, in early post-pachytene nurse cells the typical polycomplex structures, formed in these cells from the synaptonemal complex, were found in nuclear matrix preparations. The results suggest an association of synaptonemal complexes with the nuclear matrix.
-
(1984) FEBS Letters. 171, 1, p. 107-110 Abstract
The expression of cytokeratins and vimentin was investigated in epithelial cells under conditions of varied cell spreading and cell-cell contact. When extensive cell-cell contact was achieved in dense monolayer cultures, or in suspension in multicellular aggregates, the cells synthesized high levels of cytokeratins and low levels of vimentin. In contrast, sparse monolayer and suspension cultures, with minimal cell-cell contact, synthesized low levels of cytokeratins and high levels of vimentin. The ratio of cytokeratin to vimentin synthesis was independent of the cell cycle and was also reflected at the level of mRNA translational activity in vitro. Thus control of cytokeratin synthesis involves cell-cell contact, while vimentin synthesis responds to cell shape changes.
-
(1984) Journal of Molecular Biology. 172, 4, p. 467-487 Abstract
Isolated nuclei derived from simian virus 40 (SV40)-infected cells and incubated with[α-32P]UTP can elongate the in vivo preinitiated SV40 late RNA, synthesizing a viral RNA species 94 nucleotides long (attenuator RNNA) as well as longer RNA molecules. In contrast to newly synthesized SV40 RNA, the attenuator RNA is not associated with the nuclear matrix. Pretreating the cells with 5,6-dichloro-1-β-ribofuranosylbenzimidazole before the incubation of isolated nuclei in vitro, enhances the accumulation of the attenuator RNA, but again it is removed from nuclei by DNase and high salt. In contrast, pretreating the cells with proflavine, an intercalating drug that interferes with RNA secondary structure, prevents the accumulation of the attenuator RNA and increases the amount of the long RNA molecules. These RNA molecules become associated with the nuclear matrix. Isolated nuclear matrices from SV40-infected cells are highly enriched intranscriptionally active ternary complexes. Thus, isolated nuclear matrices that contain from 2 to 6% of SV40 DNA are capable of synthesizing at least 35% of the viral RNA synthesized in isolated nuclei after 2 to 15 minutes incubation with [α-32P]UTP. The RNA synthesized in vitro on purified nuclear matrices and isolated nuclei is derived from the same regions of the viral genome, suggesting that there is an association between transcribed DNA sequences and the nuclear matrix. The results suggest a major role for the nuclear matrix in controlling SV40 gene expression.
-
Cell-cell interaction and cell shape related control of intermediate filament protein synthesis(1984) Molecular biology of the cytoskeleton. Cleveland D. W., Murphy D. B. & Borisy G. G.(eds.). Cold Spring Harbor, N.Y.: . p. 435-444 Abstract
-
(1984) Mechanisms of Viral Pathogenesis: From Gene to Pathogen Proceedings of 28th OHOLO Conference, held at Zichron Yaacov, Israel, March 2023, 1983. Kohn A. & Fuchs P.(eds.). Boston, MA: . p. 1-48 (trueDevelopments in Molecular Virology). Abstract
Viral structural proteins self-assemble to produce the capsid of the virion. For an efficient self-assembly process the structural proteins should be synthesized in optimal amounts. This could be accomplished by a mechanism which somehow couples transcription in the nucleus to translation in the cytoplasm. A mechanism of gene regulation which couples transcription and translation, termed \u201cattenuation\u201d exists in procaryotes. This control is manifested through the synthesis of a small \u201cleader\u201d peptide. Successful synthesis of this peptide leads to transcription termination, whereas in the absence of its synthesis, the RNA polymerase is allowed to continue transcription through the structural genes that follow the DNA sequence coding for the leader peptide. In the present communication we summarize the available information concerning transcription termination and attenuation in eucaryotes and we show that a mechanism resembling attenuation in procaryotes regulates the production of VP1, VP2 and VP3 in SV40 and gene expression in other animal viruses. In analogy to procaryotes the leader protein of SV40 late RNA (\u201cagnoprotein\u201d) stabilizes the RNA conformation, stem-and-loop structure followed by us, which leads to transcription-termination, while deficiency of the agnoprotein leads to stabilization of an alternative RNA conformation which allows the RNA polymerase to continue transcription through the structural genes. These observations show that RNA polymerase II responds to a transcription-termination signal similar to that to which the procaryotic polymerase responds and they are included in a model in which a feedback control mechanism regulates the transcription of the viral mRNAs in the nucleus and the translation of their encoded proteins in the cytoplasm. The model has striking similarities to the attenuation model in amino acid biosynthetic operons of bacteria suggesting that SV40 has exploited a procacryotic control mechanism and adjusted it to the eucaryotic environment.
-
(1984) Molecular and Cellular Biology. 4, 9, p. 1880-1889 Abstract
Keywords: Biochemistry & Molecular Biology; Cell Biology
-
(1984) Journal of Cell Biology. 99, 4 I, p. 1424-1433 Abstract
The expression of cytokeratins and vimentin was investigated in Madin-Darby bovine epithelial cells (MDBK) in culture under conditions of varied cell spreading and cell-cell contact. When extensive cell-cell contact was achieved by seeding cells at high density in monolayer, or in suspension culture in which multicellular aggregates formed, the cells synthesized high levels of cytokeratins and low levels of vimentin. In contrast, in sparse monolayer and suspension cultures where cell-cell contact was minimal, the cells synthesized very low levels of cytokeratins. The level of vimentin synthesis was high in sparse monolayer culture and was low in both sparse and dense suspension cultures. The ratio of cytokeratin to vimentin synthesis was not affected during the cell cycle, or when cell growth was inhibited by ara C and in serum-starvation-stimulation experiments. The variations in the synthesis of cytokeratins and vimentin under the various culture conditions were also reflected at the level of mRNA activity in a cell-free in vitro translation system and as determined by RNA blot hybridization with cDNA to vimentin and cytokeratins. The results suggest that control of cytokeratin synthesis involves cell-cell contact, characteristic of epithelia in vivo, while vimentin synthesis responds to alterations in cell spreading.
-
(1984) International Journal of Cancer. 33, 1, p. 115-121 Abstract
The ability of retinoic acid to modulate cellshapedependent growth of untransformed (human skin fibroblasts and mouse embryo Swiss 3T3 fibroblasts) and neoplastic cells (human cervical carcinoma HeLaS3, osteosarcoma Hs791, and murine melanomas B16F1, S91C2 and S91C154) was examined. The cells were plated on tissue culture dishes coated with increasing concentrations of poly(2hydroxyethyl methacrylate), poly(HEMA) which cause a gradual decrease in substrate adhesiveness. Untreated cells as well as cells pretreated with 10 μM retinoic acid for 4 days displayed a similar graded series of cell shapes between flat and spherical on these modified substrata, with the exception of HeLaS3 cells which were rounded and loosely attached even on uncoated plastic dishes. A marked cellshapedependent decrease in DNA synthesis was observed in untransformed human skin fibroblasts, Swiss 3T3 fibroblasts and neoplastic human Hs791 cells 20 h following plating of untreated cells on poly(HEMA)coated substrates of decreasing adhesiveness. Conversely, in B16F1, HeLaS3 and S91C154 cells DNA synthesis was only slightly affected by changes in cell shape. Pretreatment with retinoic acid rendered DNA synthesis in Swiss 3T3, Hs791, B16F1 and S91C2 cells much more sensitive to changes in cell shape. In contrast, retinoic acid exerted only marginal effects on the sensitivity of DNA synthesis to changes in cell shape in untransformed human skin fibroblasts, in HeLaS3 cells and in the retinoicacidresistant S91C154 cells. The results suggest that retinoic acid can restore in certain tumor cells the tight coupling between cell shape and DNA synthesis that exists in untransformed cells.
-
(1983) Virology. 129, 2, p. 501-507 Abstract
Triton cytoskeletons and nuclear matrices were prepared from herpes simplex virus (HSV)-infected cells by a sequential fractionation scheme. Electron microscopic studies revealed the association of mature HSV with the filamentous network of the nuclear matrix. Indirect immunofluorescence assays with monoclonal antibodies revealed that ICP5, the major capsid protein, accumulated on the nuclear matrix while ICP8, the major viral DNA binding protein, accumulates in the chromatin fraction that can be separated from the nuclear matrix by extraction with DNase and salt. Pulse-chase experiments confirmed the kinetics studies of D. M. Knipe and A. E. Spang (J. Virol.43, 314324, 1982) and showed that ICP5 is transported after a lag from the cytoplasmic framework to the nuclear matrix, while ICP8 is transported faster to the chromatin fraction.
-
(1983) The EMBO Journal. 2, 7, p. 1041-1047 Abstract
The subcellular localization of the p53 molecule was studied in transformed and nontransformed fibroblasts. A newly established transformed cell line obtained by treating primary embryonic mouse cells in vitro with the chemical carcinogen methylcholanthrene was compared with the embryonic parent fibroblasts. The transformed cells lost the spindle shape characteristic of the parent fibroblasts, acquired an accelerated growth rate, developed into tumors when injected into syngeneic mice and expressed high levels of p53 synthesis estimated by immunoprecipitation of [35S]methioninelabeled cell extracts. The cellular localization of the p53 molecule was studied by immunofluorescent staining of fixed cells with monoclonal antibodies and by immunoprecipitation of [35S]methioninelabeled p53 from various subcellular fractions. p53 was mainly found in the nucleus of the transformed fibroblast, while in the parent nontransformed primary embryonic cells, p53 was detected in the cytoplasm in a Triton X100 soluble fraction, and associated with the cytoskeleton. The modulated distribution of p53 was also confirmed by analyzing a wide range of independently established transformed and nontransformed fibroblastic cell lines growing in vitro. The switch from the cytoplasmic localization of p53 in the nontransformed fibroblasts to a chromatinassociated accumulation in the transformed cells suggests a possible mechanism by which this protein may function in the transformed fibroblasts.
-
(1983) Virology. 125, 2, p. 475-479 Abstract
Nuclear matrices from SV40-infected cells were prepared by treating purified nuclei with DNase and salt to extract DNA and histones. After a 10-min pulse with [5,6-3H]uridine over 85% of the viral RNA was found in association with the nuclear matrix. Following a 3-hr chase with glucosamine and unlabeled uridine, 24 S nuclear viral components accumulate, but they are not associated with the nuclear matrix. The 24 S components had been characterized previously as viral RNA processing products (N. H. Chiu, M. F. Radonovich, M. M. Thoren, and N. P. Salzman, J. Virol. 28, 590599, 1978). The results of the present study identify the 24 S components as DNA rather than RNA, thus indicating that the synthesis and the majority of the processing products of the viral RNA are associated with the nuclear matrix.
-
Virus replication in infected epithelial cells is coupled to cell shaperesponsive metabolic controls(1983) Journal of Cellular Physiology. 114, 2, p. 145-152 Abstract
The cell shape of monkey epithelial cells was varied from flat to spheroidal by gradually reducing the substrate adhesiveness with poly (HEMA) films of increasing thickness. The decrease in cell spreading is accompanied by a dramatic response in cellular macromolecular metabolism in the nucleus. Within 14 to 16 hr, DNA and RNA syntheses are inhibited by more than 95%, while the level of protein synthesis is reduced by only twofold after 24 hr in spheroidalsuspension culture. When epithelial cells, spread to various degrees, are infected with SV40 or herpes virus a parallel inhibition of virus replication and cellular macromolecular metabolism occurs. However, VSV can proliferate in the metabolically active cytoplasm of epithelial cells in which nuclear activity is inhibited owing to alterations in cell shape. The results suggest that the metabolic restrictions imposed on epithelial cells, owing to changes in cell spreading, are a dominant phenomenon that cannot be overcome by virus infection. Rather, virus replication, which is dependent on the cellular metabolic machinery, is inhibited in parallel with the inhibition of cellular macromolecular metabolism.
-
(1983) Molecular and Cellular Biology. 3, 2, p. 182-189 Abstract
The role of cell configuration in regulating cell metabolism has been studied, using a system in which cell shape and surface contact can easily be manipulated. The suspension of anchorage-dependent mouse fibroblasts in Methocel results in a coordinate decrease of DNA, RNA, and protein synthesis. These processes are restored upon reattachment of cells to a solid surface. This recovery process has two or more components: a rapid recovery or protein synthesis requiring only surface contact, and a slower restoration of nuclear events which is dependent upon extensive cell spreading. In the present study, we examined 3T3 cells while in suspension culture and after attachment to a tissue culture dish surface to study cell configuration-dependent expression of specific cytoskeleton protein genes. The 3T3 line of fibroblasts used here shows these responses much more dramatically compared with 3T6 cells previously studied. We demonstrate that whereas total protein synthesis was strongly inhibited upon suspension, actin synthesis was preferentially inhibited, decreasing from 12% of total protein synthesis in control cells to 6% in suspended cells. This occurred apparently at the level of translation of actin mRNA, since the amount of actin mRNA sequences in the cytoplasm was unchanged. Reattachment initiated the rapid recovery of overall protein synthesis which was accompanied by a dramatic, preferential increase in actin synthesis reaching peak values of 20 to 25% of total protein synthesis 4 to 6 h later, but then declining to control values by 24 h. Translation in vitro and hybridization of mRNA to a cloned actin cDNA probe revealed that the induction of actin synthesis was due to increased levels of translatable mRNA cytoarchitecture, expression of a specific cytoskeletal protein gene, and growth control. The expression of the actin gene appears to be regulated at both the level of translation (during suspension) and mRNA production (during recovery).
-
(1983) Journal of Cell Biology. 97, 3, p. 858-865 Abstract
The cell configuration-related control of a cytoskeletal protein (vimentin) expression was examined by varying cell shape between flat and spherical. Cultivation of cells in monolayer or in a spherical configuration on poly-2-hydroxyethylmethacrylate-coated plates revealed a preferential down regulation of vimentin synthesis during suspension culture. The mechanism(s) regulating the decrease in the expression of vimentin in spherical cells appears to be at the level of translation, because mRNAs extracted from monolayer and suspension-cultured cells were equally active in directing vimentin synthesis in the rabbit reticulocyte cell-free system. When after prolonged suspension culture, the cells were allowed to reattach and spread, vimentin synthesis recovered rapidly to the control monolayer rate. The phosphorylation of vimentin was also reduced dramatically during suspension culture. However, unlike the rapid recovery of cimentin biosynthesis upon reattachment (20 h) and paralleled the recovery to the monolayer growth rate. Although the control of vimentin biosynthesis in suspension culture is a cell configuration-related process, the decrease in the rate of vimentin phosphorylation in suspension culture appears to be the result of the slower growth rate and may reflect the reported correlation between the rate of vimentin phosphorylation and the accumulation of cells in mitosis.
-
(1983) Science. 221, 4617, p. 1307-1310 Abstract
The lung colonization of B16-F1 cells grown in flat and spherical configurations was studied. Cells cultivated in vitro as spheroids on a nonadhesive substrate expressed in a reversible fashion a marked increase in their propensity to establish metastases. The altered metastatic capability was accompanied by a reversible reduction in the accessibility of cell surface proteins to external iodination and by a dramatic decrease in the synthesis of vimentin.
-
(1983) Molecular and Cellular Biology. 3, 4, p. 684-692 Abstract
Keywords: Biochemistry & Molecular Biology; Cell Biology
-
(1982) International Journal of Cancer. 29, 6, p. 711-715 Abstract
Growth control and sensitivity to changes in cell shape were studied in anchoragedependent mouse fibroblasts (diploid fibroblasts, 3T3 and 3T6), in DNA tumorvirustransformed mouse fibroblasts (SVPy 3T3), in four B16 melanoma and five uv2237 fibrosarcoma cell variants that exhibit distinct metastatic properties. Differential adhesive conditions were established by precoating the plastic plates with poly (2hydroxyethylmethacrylate) that allowed an accurate and reproducible control of cell shape, from flat to spherical. Mouse fibroblasts that form a continuum between rigorously controlled cells to fully anchorageindependent cells, display a direct correlation between degree of growth control and sensitivity to changes in cell spreading. In contrast, there is no apparent direct correlation between sensitivity of growth control to changes in cellular configuration and the metastatic potential of tumor cells.
-
(1982) The EMBO Journal. 1, 10, p. 1225-1231 Abstract
Nuclear matrices were prepared by DNase and high salt extraction of SV40-infected epithelial monkey cells. The matrices retain the majority of SV40 virions. This conclusion is based on electron microscopic observations of the occurrence of encapsidated viral DNA that is resistant to DNase digestion and on the analysis of viral proteins by gel electrophoresis. Pulse labeled SV40 RNA is also associated with the nuclear matrix (less than 15% of the viral RNA is removed by DNase and high salt). Pulse-chase experiments revealed that processing of SV40 RNA takes place on the nuclear matrix and the processed molecules are directly transported to the cytoplasm where they are associated with the cytoskeleton. These results suggest a central role for the nuclear and cytoplasmic substructures in virus assembly and in the biogenesis of viral RNA.
-
(1981) Cell Biology International Reports. 5, 12, p. 1127-1135 Abstract
Addition of cationized ferritin to Triton X-100 extracted fibroblasts enhances manyfold the visibility of actin filaments, intermediate filaments and microtubules. Cationized ferritin also increases the phase image of actin filaments in skeletal muscle myofibrils from which myosin has been removed, and the phase contrast of Z-bands after extracting both myosin and actin from myofibrils. It is suggested to employ this non-specific reagent as a rapid technique for the visualization of cytoskeletal elements in cells.
-
(1981) Cell. 26, 1 PART 1, p. 107-115 Abstract
Cell proliferation under nonadhesive conditions was examined in anchorage-dependent mouse fibroblasts (3T3 and 3T6), in epithelial monkey kidney cells (BSC-1) and in four B16 melanoma cell variants that exhibit distinct metastatic properties. Non-adhesive conditions established by suspension culture in methyl cellulose or by plating on poly(2-hydroxyethylmethacrylate)-coated plates cause multinucleation and inhibition of cytokinesis in all cell types. Formation of binucleated cells in suspended 3T3 cells continues for 24 hr until DNA synthesis is completely inhibited. Twenty percent of the cells become binucleated during this time. During a similar period in suspension culture over 60% of a synchronized epithelial cell culture (suspended 8 hr after the initiation of S phase) became binucleated. The melanoma cells that maintain a high level of metabolism while in suspension continued to develop into multinucleated cells. After 3 days in suspension, 50% of the melanoma cells have more than one nucleus. Since cell number during suspension culture remains constant, because of a block in cytokinesis, there is a remarkable increae in cell size in suspended melanoma cells. When replated on tissue culture plates, normal cell division resumes by an early extrusion of the extra nuclei in melanoma cells. The melanoma cell variants, although displaying different metastatic potential in vivo, behave similarly in suspension culture. 3T3 cells require 24 hr of replating before recovering DNA synthesis and initiating extrusion of the extra nucleus. The results suggest a differential sensitivity of cytokinesis and karyokinesis to cell-surface contact with the substrate.
-
(1981) Virology. 111, 2, p. 475-487 Abstract
We prepared the cytoskeletal framework of SV40-infected BSC-1 cells late after infection by extracting the cells with Triton X-100. The cytoskeletal framework obtained by this procedure carries more than 80% of the newly synthesized viral polyribosomes. Almost all the viral-specific cytoplasmic RNA is engaged in the formation of cytoskeletal-associated polyribosomes. The cytoskeletal framework harbors both the poly(A)+ 19 S and 16 S late viral RNAs, as well as most of the poly(A)- viral RNA. The poly(A)- viral RNA sediments in sucrose gradients between 4 and 18 S representing heterogeneous species and comprises about 30-50% of the total virus-specific RNA in the cytoplasm. Based on pulse-chase experiments it is concluded that: (i) the poly(A)+ viral RNA emerges from the nucleus and becomes associated with the cytoskeletal framework, (ii) the decay rate of the poly(A)+ RNA species on the cytoskeletal framework is faster than that of the poly(A)- viral RNA, and (iii) both the poly(A)+ and poly(A)- viral RNAs detach from the cytoskeletal framework and move to the soluble fraction.
-
-
Altered translatability of messenger RNA from suspended anchorage-dependent fibroblasts: reversal upon cell attachment to a surface(1981) Readings in Mammalian Cell Culture. Pollack R.(eds.). 2 ed. Cold Spring Harbor, NY: . p. 289-299 Abstract
-
The outer boundary of the cytoskeleton: a lamina derived from plasma membrane proteins(1981) Readings in Mammalian Cell Culture. Pollack R.(eds.). 2 ed. Cold Spring Harbor, NY: . p. 642-648 Abstract
-
(1980) Journal of Cellular Physiology. 103, 2, p. 247-254 Abstract
Suspending anchoragedepent fibroblasts in methocel results in marked reduction in a number of macromolecular metabolic processes. These are restored when cells make contact with a solid substrate and are allowed to spread. The response of 3T6 cells to suspension culture and their recovery upon reattachment has been extensively studied. (Benecke et al., '78; Farmer et al., '78). In these cells, message production and its turnover rate both drop abruptly when cells are suspended, while protein synthesis declines very slowly but extensively. Recovery of protein synthesis in 3T6 cells only requires surface contact and not cell spreading and proceeds rapidly, reaching control values within a few hours. The recovery of messenger RNA production in spread cells is much slower, and commences about 18 hours after cells are replated.The present report extends the studies on RNA regulation in suspended and reattached 3T6 fibroblasts. All RNA synthesis systems respond to cell suspension. Production of small nuclear RNA species is shown to behave very similarly to the production of messenger RNA, suggesting these may be coordinately regulated. Ribosomal precursor RNA synthesis ceases promptly upon cell suspension and recovers only slowly after reattachment. The control of ribosome formation appears independent of the regulation of protein synthesis in this system. The products of polymerase III including transfer RNA, 5S RNA, and species K and L are all regulated together and show a distinct pattern of behavior unlike that of any other RNA species. Their formation is rapidly inhibited at the beginning of suspension, but then recovers to the level of control cells, even during continued suspension culture. This unusual property of polymerase III RNA species is quite different from the behavior of the small nuclear (snRNA) RNA species, which strengthens the previous suggestion that these two classes of RNA molecules are formed by different mechanisms and regulated independently.
-
(1980) Cell. 21, 2, p. 365-372 Abstract
Anchorage-dependent mouse fibroblasts grow only if attached to and spread on a solid substrate. The suspension of cells in methyl-cellulose results in dramatic, coordinated inhibition of the major RNA and protein synthesis systems, and these systems are sequentially restored when cells are replated on a tissue culture dish surface. In the present report the effects on metabolism of cell reattachment are separated from those of subsequent spreading by controlling cell shape. Macromolecular metabolism is first strongly suppressed by long-term suspension culture. The cells are then replated in the presence of a variety of spreading inhibitors. The recovery of protein synthesis, which rapidly follows reattachment, does not require extensive cell spreading. Contact of a limited portion of the plasma membrane with the solid culture dish surface is apparently a sufficient signal by itself. A very different method of controlling cell shape is afforded by changing culture dish surface adhesivity. Suspended cells are replated on dishes precoated with thin layers of the hydrophilic hydrogel poly(2-hydroxyethyl methacrylate). The final mean cell diameter is then varied over wide limits. As before, protein synthesis recovery is unaffected. However, nuclear events such as DNA and rRNA synthesis and mRNA production are profoundly affected by cell shape. Thus, cell surface contact and cell shape give rise to distinctly different regulatory responses.
-
(1979) Cell. 17, 2, p. 319-325 Abstract
Colchicine and nocadazole both depolymerize microtubules in cultured fibroblasts and lead to a rapid inhibition of tubulin synthesis. The level of translatable tubulin mRNA is greatly reduced in drug-treated cells as demonstrated by translation in a reticulocyte-derived in vitro protein synthesizing system. A model of tubulin synthesis regulation is proposed in which the elevated level of unpolymerized tubulin in drug-treated cells inhibits the formation of new tubulin mRNA and the preexisting message decays rapidly. In agreement with this model, tubulin message is found to be short-lived and has an approximately 2 hr half-life in cells treated with actinomycin D. Another prediction of the proposed model is that destabilization of microtubules without a concomitant increase in free tubulin will not inhibit tubulin synthesis. Vinblastine also disrupts microtubules but leads to the aggregation of tubulin into large paracrystals with an apparent decrease in the concentration of free tubulin. This drug does not inhibit tubulin production but rather leads to a measurable enhancement of tubulin synthesis.
-
(1979) Cell. 17, 4, p. 859-865 Abstract
We prepared the cytoskeletal framework by gently extracting cells with Triton X-100. Lipids and soluble proteins were removed, leaving a complex meshlike structure which contains the cell nucleus and is composed of the major cell filament networks as well as the microtrabeculae with attached polyribosomes. The surface sheet or lamina covering this structure contains most of the cell surface proteins by the following criteria. Intact cells are labeled externally with radioiodine and then extracted with detergent. The iodinated proteins remain almost entirely with the skeletal framework. A new major integral protein, the coat protein of Sindbis virus, is inserted into the plasma membrane of infected cells. This new protein is heavily iodinated and remains almost completely associated with the framework after extraction. Lectin binding and poliovirus binding sites are also retained after detergent extraction. Our results indicate that plasma membrane proteins form a sheet or lamina upon removal of lipids. This lamina reproduces even complex surface convolutions and appears to be supported by and intimately connected to the underlying skeleton. In this case, the surface lamina, and hence the plasma membrane of the original intact cell, might be viewed as a component of the cytoskeletal framework.
-
(1978) Archives of Virology. 58, 1, p. 71-76 Abstract
Transcription of virus DNA was detectable in vitro in nuclei isolated from cells infected with wild type and bromodeoxyuridine (BUdR)-resistant herpes simplex virus type 1 but was suppressed in nuclei of cells infected with ultraviolet irradiated or defective virus or cells treated with BUdR during infection.
-
(1978) Cell. 14, 4, p. 931-939 Abstract
Strictly anchorage-dependent 3T6 fibroblasts require contact with a solid surface for growth and for normal macromolecular metabolism. Denial of surface contact or anchorage by suspension in methocel leads to dramatic changes in messenger RNA metabolism and protein synthesis. These are reversed when cells contact a surface, even when spreading is prevented by agents such as concanavalin A. The responses to suspension appear to be well ordered regulatory events. While the synthesis of hnRNA remains constant, the production of mRNA is reduced 5 fold within a few hours under suspension conditions, suggesting a possible post-transcriptional control mechanism. Normally turning over messenger RNA is stabilized; the breakdown rate is reduced to match the lowered rate of message production so that the total amount of mRNA in the suspended fibroblasts remains constant. Protein synthesis declines slowly but extensively, and amounts to only 15% of control cells after 72 hr of suspension. The rate of protein synthesis is clearly not regulated by the amount of mRNA present, but rather by its availability. This is shown by the 6-7 fold increase of protein synthesis which occurs within 4 hr after the reattachment of suspended cells to a solid substrate. Messenger RNA production remains low during this period and cannot account for the resumption of protein synthesis. Rather, the recovery utilizes preexisting mRNA that has been translationally inactive, and this mRNA, once returned to translation, is engaged on full-sized polyribosomes, indicating the absence of a lesion in polypeptide initiation. At least one major protein, of about 43,000 dalton molecular weight, shows greatly enhanced synthesis during recovery. This polypeptide comigrates with actin in two-dimensional gel electrophoresis and is extensively associated with the cytoskeleton. Its enhanced synthesis following cell reattachment may represent a modulation of a morphology-related gene.
-
(1978) Cell. 15, 2, p. 627-637 Abstract
Anchorage-dependent cells, when forced into suspension culture, display a repertoire of dramatic, coordinated regulatory phenomena. Message production promptly decreases 5 fold but the cells maintain a constant amount of poly(A)+ by means of a concomitant stabilization of mRNA against decay. Protein synthesis shuts down much later and the mRNA is stored in a nonfunctioning state. In this study, the inactive mRNA is extracted from suspended cells and shown to have aberrant translation properties. Well defined polypeptides are apparently no longer synthesized when this mRNA directs protein formation in either reticulocyte or wheat germ-derived heterologous translation systems. Rather, shortened peptides are formed by this mRNA and these become smaller as mRNA is used from cells suspended for longer periods of time. Very few focused spots are formed when the aberrant polypeptides are analyzed in two-dimensional electrophoresis. The sedimentation properties of suspended cell mRNA and the size of poly(A) are unchanged from control monolayer cells. Cross-hybridization of cDNA transcribed from a control cell message population with suspended cell mRNA shows that all sequences are present in normal concentrations. While most identifiable spots disappear from the two-dimensional gel electropherograms of the protein products produced by suspended cell mRNA, a few polypeptides are still synthesized in relatively normal amounts. Conserved polypeptides are found in products of both the reticulocyte and wheat germ systems, but they are different products in each case. The lesion in the suspended cell mRNA does not seem to be at the 5 termini, since synthesis of the shortened peptides is fully sensitive to inhibition by pm7G. Cells that contain extensively modified message can resume protein synthesis when allowed to reattach to a solid substrate. There is an apparent remodification of mRNA to normal translatability within a few hours of cell reattachment, since mRNA from recovering cells quickly resumes directing relatively normal patterns of polypeptide synthesis in vitro. The restoration of normal message function occurs even when new message formation is blocked with actinomycin. Cells recovering after reattachment synthesize supranormal amounts of a few major proteins involved with cell structure, as shown in these studies by an increased amount of translatable sequences which encode these proteins. The most apparent enhanced message is that coding for actin. mRNA from recovering cells produces in vitro several times more actin relative to other proteins than does control cell mRNA. The enhancement of actin mRNA is not seen in the message population of cells that reattach in the presence of actinomycin. The results suggest a morphologically related induction of gene expression.
-
(1977) Virology. 76, 1, p. 246-253 Abstract
The replication of herpes simplex virus (HSV) in α-amanitin-sensitive (BSC-1 and BHK-21) and -resistant (α-amr, BHK-T6-G1) cell lines was studied in the presence and absence of α-amanitin. HSV replicates in the resistant cell line in the presence of α-amanitin, but fails to do so in α-amanitin-treated BSC-1 and BHK-21 cells. It was concluded that the host cell RNA polymerase II is required for the replication of HSV.
-
Isolation of Marek's disease virus DNA from infected cells by electrophoresis on polyacrylamide gels(1977) Archives of Virology. 54, 1-2, p. 75-83 Abstract
Marek's disease virus DNA isolated from the nuclear fraction of infected chicken embryo fibroblasts and sucrose-purified particles was electrophoresed on 3 per cent polyacrylamide gels and was compared in its electrophoretical behaviour with isolated pseudorabies and herpes simplex DNA, strain HF. The DNA molecules eluted from the gel were identified by their sedimentation coefficient (53--55S) and buoyant density (1.707 g/ml) to be of viral origin. MDV DNA molecules were electrophoretically also detected and identified in DNA preparations of the lymphoblastoid Marek's disease tumour cell line MSB-1 which therefore has to be considered as a producer line. The electrophoresis of DNA preparations from Marek's disease virus-infected cells on polyacrylamide gels provides a semipreparative method for the isolation of MDV DNA.
-
(1976) Virology. 71, 1, p. 302-11 Abstract
Nuclei isolated from herpes simplex virus (HSV)-infected BSC-1 cells synthesize virus-specified RNA by an RNA polymerase II that is sensitive to 0.4 μg/ml of α-amanitin. The synthetic activities in infected and uninfected nuclei differ in their reaction kinetics, salt requirements, and the extent of RNA synthesis as a function of temperature. When the cells are infected in medium lacking arginine, the synthetic activity is reduced by 50% in the infected nuclei but remains unchanged in nuclei from arginine-deprived uninfected cells. The synthetic activity specified by the virus is closely related to the virus growth cycle. The RNA synthesized is heterogenous in size (428 S) and hybridizes efficiently with HSV DNA. In the presence of 0.4 μg/ml α-amanitin, mainly low molecular weight RNA (45 S) is synthesized. Some of this RNA hybridizes to HSV DNA.
-
(1975) Nature. 254, 5502, p. 719-22 Abstract
ALONI13 reported that late in the replicative cycle of polyoma and simian virus 40 (SV40) viruses the viral DNA genomes are symmetrically transcribed in the infected cells. The newly synthesised RNA molecules labelled for very short periods were shown to be complementary and on self-annealing yielded high molecular weight double stranded (ds) RNA. Aloni and Attardi showed that similar symmetrical transcription occurs in HeLa cell mitochondria4,5. In further studies Aloni2 demonstrated that after polyadenylation of the SV40 symmetrical RNA transcripts the RNA is processed to yield uncomplementary viral mRNA molecules.
-
Nucleic acid biosynthesis in nuclei of cell infected with herpesviruses (HSV and EBV)(1975) IARC scientific publications. No.11 I, 11 Pt 1, p. 245-57 Abstract
Analysis of nuclei isolated from herpes simplex virus (HSV)-infected cells by electrophoresis in polyacrylamide gels showed that only virion-associated DNA molecules migrated into the gels. The viral DNA molecules, which do not migrate in the gels, are the precursors for the mature viral DNA. Centrifugation of deoxycholate-treated infected nuclei in sucrose gradients revealed that most of the viral DNA co-sedimented with the cellular DNA. The DNA-dependent RNA polymerase activity also co-sedimented with the viral and cellular DNA. Incubation of nuclei isolated from HSV-infected cells, under in vitro conditions that support DNA synthesis, resulted in the synthesis of viral molecules of low molecular weight. Under the same conditions, nuclei from Burkitt lymphoblasts did not synthesize DNA. The nature of the association between cellular DNA and EBV DNA was studied by hybridization with EBV cRNA. EBV-specific sequences were found in the lymphoblast DNA; they banded at a density of 1.707 g/cm3. Some of the viral mRNA transcribed in HSV-infected nuclei is symmetrically transcribed from HSV DNA. The symmetrical portions of the RNA are removed, since poly (A)-containing mRNA molecules lack homologous sequences. Most of the RNA synthesized in HSV-infected nuclei can be released after incubation of the nuclei in vitro in the presence of ATP. The released RNA consists of poly(A)plus and poly (A)-minus molecules. The mechanism of RNA transport from the nuclei still remains to be studied.
-
Integration of Epstein-Barr virus DNA into cellular DNA of Burkitt lymphoblasts (Raji cell line).(1974) Israel Journal of Medical Sciences. 10, 11, p. 1454-1457 Abstract
-
(1974) Journal of General Virology. 25, 1, p. 63-73 Abstract
The DNA molecules synthesized in herpes simplex virus (HSV) infected cells were analysed by electrophoresis in polyacrylamide gels. It was found that part of the virus DNA, isolated from infected nuclei, migrated into the gels in a manner similar to the virus DNA molecules isolated from purified herpes simplex virus particles. Most of the virus DNA molecules, labelled in vivo for 60 min with [3H] thymidine as well as labelled cellular DNA molecules, were retained at the top of the gel during electrophoresis. The amount of HSV DNA which migrated into the gel gradually increased when the labelled cells were reincubated after removal of the isotope (chase). Most of the DNA molecules, which were retained at the top of the gel, had the density of virus DNA but differed in their sedimentation behaviour in sucrose gradients from the intact DNA molecules isolated from purified virus particles. The presence of replicating as well as mature virus DNA molecules in infected nuclei is discussed.