Interaction of senescent cells with the immune system
- We study the mechanisms of interaction of senescent cells with immune cells and harness these mechanisms for the elimination of senescent cells. We have discovered mechanisms of interaction of senescent cells with components of both innate and adaptive immune systems.
- Immune checkpoint regulates immune surveillance of senescent cells:
We found that p16-positive senescent cells upregulate the immune checkpoint protein programmed death-ligand 1 (PD-L1) to accumulate in ageing and chronic inflammation. p16-mediated inhibition of cell cycle kinases CDK4/6 induces PD-L1 stability in senescent cells via downregulation of its ubiquitin-dependent degradation. p16-expressing senescent alveolar macrophages elevate PD-L1 to promote an immunosuppressive environment that can contribute to an increased burden of senescent cells. Treatment with activating anti-PD-L1 antibodies engaging Fcγ receptors on effector cells leads to the elimination of PD-L1 and p16-positive cells. Our study uncovers a molecular mechanism of p16-dependent regulation of PD-L1 protein stability in senescent cells and reveals the potential of targeting PD-L1 to improve immunosurveillance of senescent cells and ameliorate senescence-associated inflammation (Majewska et al, Nature Cell Biology 2024).
Senescent cells – PDL-1 movie »
-
Aging goes hand in hand with the progressive dysregulation of biological systems, including immune responses. Natural killer (NK) cells are potent component of the innate immune system that play an important role in the immune surveillance of senescent cells.
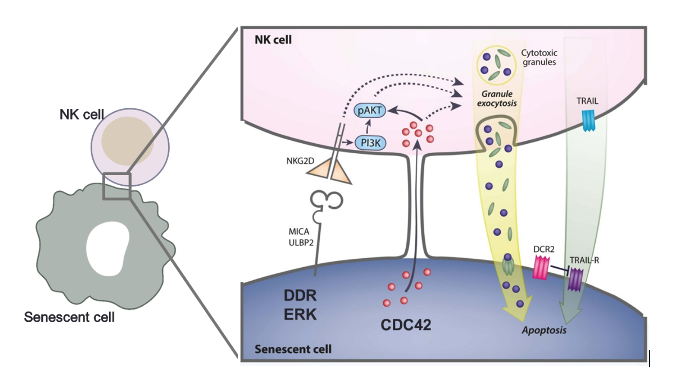
The immune surveillance of senescent cells by NK cells requires three essential steps: attraction of NK cells by senescent cells to their vicinity, recognition of senescent cells through specific receptor-ligand interaction that initiates the cytotoxicity of NK cells, and finally, formation of immune synapse and killing of senescent cell. Components of senescence-associated secretory phenotype (SASP) attract the NK cell. We have previously discovered that NK cells recognize senescent cells through the NKG2D receptor and eliminate them by granule exocytosis-mediated mechanism (Sagiv et al, 2013, Sagiv et al, 2016). Apparently, senescent cells also communicate with NK cells through tunneling nanotubes in CDC42-dependent manner (Biran et al, Genes&Development 2015). We have shown that impairment of this elimination leads to accumulation of senescent cells and accelerated aging (Ovadya et al, Nature Communication 2018).

