Senescence in aging & age-related diseases
Senescent cells accumulate in the organism with age, contributing to age-related diseases and aging.
Cellular senescence, a permanent state of cell cycle arrest accompanied by a complex phenotype, is an essential mechanism that limits tumorigenesis and tissue damage. In physiological conditions, senescent cells can be removed by the immune system, facilitating tumor suppression, wound healing, and embryonic development. However, as we age, senescent cells accumulate in tissues, either because an aging immune system fails to remove them, an increase in the rate of senescent cell formation, or both. If senescent cells persist and accumulate in tissues, they promote pathological conditions and age-related diseases, some of which are the focus of our research.
Senescence in age-related diseases
Chronic lung inflammation
 Chronic inflammation is strongly associated with aging and has even been referred to as 'inflammaging”. The lung is one of the organs where chronic inflammation is known to contribute to age-related diseases. One such disease, Chronic Obstructive Pulmonary Disease (COPD) is characterized by persistent airflow limitation associated with an enhanced chronic inflammatory response. COPD is currently the fourth leading cause of death in the developed countries. Aging is one of the major risk factors for COPD. One emerging hypothesis suggests that alveolar- and airway-cell senescence triggered by environmental stress or aging, contributes to the destruction of alveoli. Cellular senescence can limit the proliferative capacity necessary for tissue repair and promote chronic inflammation via the senescence-associated secretory phenotype (SASP), both of which are hallmarks of COPD. We study the role of senescent cells in the pathology of COPD and other age-related inflammatory diseases using genetically modified mouse models (Sagiv et al, Cell Reports, 2018, Levi et al, Aging 2023).
Chronic inflammation is strongly associated with aging and has even been referred to as 'inflammaging”. The lung is one of the organs where chronic inflammation is known to contribute to age-related diseases. One such disease, Chronic Obstructive Pulmonary Disease (COPD) is characterized by persistent airflow limitation associated with an enhanced chronic inflammatory response. COPD is currently the fourth leading cause of death in the developed countries. Aging is one of the major risk factors for COPD. One emerging hypothesis suggests that alveolar- and airway-cell senescence triggered by environmental stress or aging, contributes to the destruction of alveoli. Cellular senescence can limit the proliferative capacity necessary for tissue repair and promote chronic inflammation via the senescence-associated secretory phenotype (SASP), both of which are hallmarks of COPD. We study the role of senescent cells in the pathology of COPD and other age-related inflammatory diseases using genetically modified mouse models (Sagiv et al, Cell Reports, 2018, Levi et al, Aging 2023).
Fibrotic diseases (Lung fibrosis, liver fibrosis)
 Aging is associated with extracellular matrix (ECM) accumulation and fibrosis, in most tissues of the mammalian organism. Senescent cells are present in human and mouse fibrotic diseases and contribute to their development.
Aging is associated with extracellular matrix (ECM) accumulation and fibrosis, in most tissues of the mammalian organism. Senescent cells are present in human and mouse fibrotic diseases and contribute to their development.
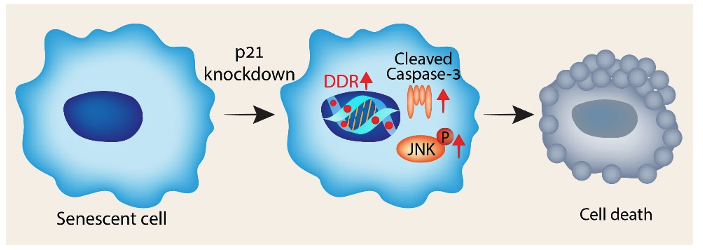
We have discovered that p21 knockout or even knockdown during fibrosis prevents disease development. Additionally, we have delineated the molecular mechanism underlying this effect (Yosef et al, EMBO J, 2017, Papismadov et al, EMBO J. 2024). These mechanisms involve the senolytic, senomorphic, and anti-fibrotic activities of p21 knockdown. We focus on understanding the mechanisms and exploring approaches to modify p21 expression and function, in order to better understand the development of fibrotic diseases and develop new approaches for their treatment.
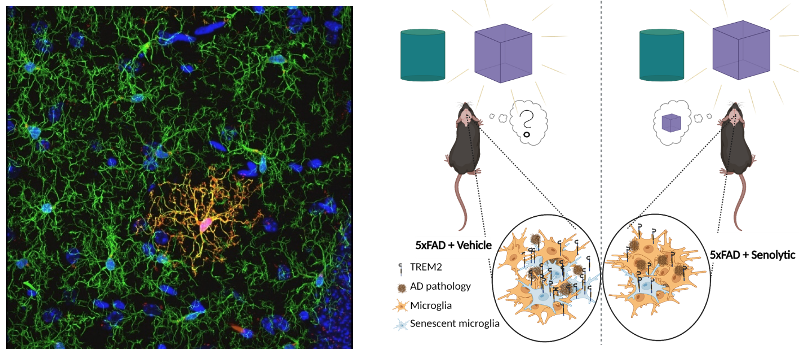
Neurodegenerative diseases
With age, senescent cells progressively accumulate in various tissues, including the brain. We aim to investigate the role of senescent cell accumulation in brain aging. We detected a distinct population of microglia exhibiting a characteristic senescent signature in the brains of aged mice and mouse models of Alzheimer’s Disease (AD). Eliminating senescent cells throughout the organism in AD mice by a senolytic treatment reduced signs of AD and alleviated memory loss, as assessed by molecular and behavioral tests (Rachmian et al, Nature Neuroscience, 2024). The conserved senescence microglial signature observed in both aging and AD, could explain the strong association between these conditions and provide new routes to slow cognitive decline during aging.