Publications
-
(2026) The Journal of Cell Biology. 225, 2, e202501134. Abstract
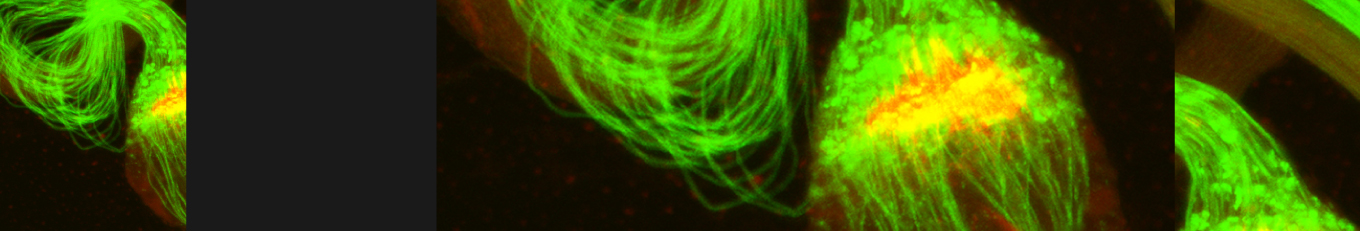
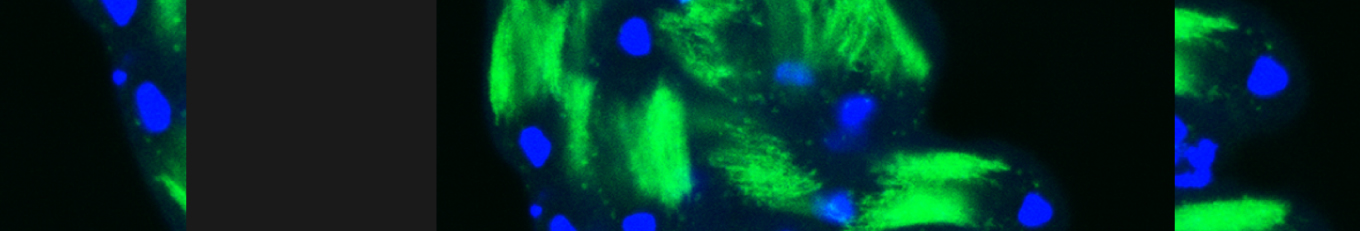
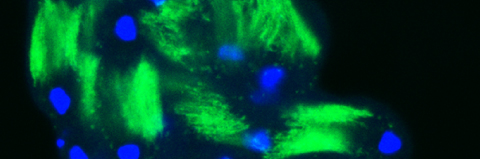
Somatic cells in both mammalian and Drosophila testes perform diverse roles in regulating germline stem cell differentiation into sperm. Beyond their supportive functions, such as encapsulation and signaling, somatic cells also act as tissue-resident, non-professional phagocytes. In Drosophila testis, somatic cyst cells eliminate approximately a quarter of newly emerged spermatogonial progenitors, a role seemingly contradictory to their supportive function. Here, we examined individual events in which cyst cells alternated between supporting germ cells and promoting their death, revealing distinct morphological features. Our data indicate that, in addition to well-defined cyst cells derived from stem cell divisions and escorting differentiating spermatogonia, a distinct population of long-lived steady cyst cells arises during larval development. These steady cyst cells persist at the apical tip of the testis for extended periods and engage in phagoptosis. This distinction separates cyst cells into two subpopulations based on function and morphology, highlighting how genetically similar cells adopt specialized roles depending on their developmental origin and timing.
-
(2025) Nature Communications. 16, 1, 10871. Abstract
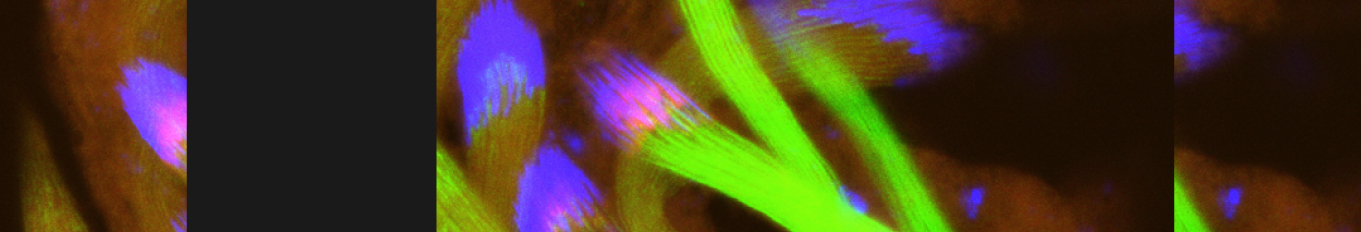
Caspases are best known for promoting apoptosis, yet their role in tissue regeneration by compensatory proliferation remains unclear. Using Drosophila wing discs and a delayed reporter for the initiator caspase-9 ortholog Dronc activity, we identify two apoptosis-resistant epithelial cell populations that mediate regeneration after ionizing radiation: Dronc-activating (DARE) and non-activating (NARE) cells. Dronc activity in DARE cells, independent of Dark and effector caspases, drives regeneration both cell-autonomously and non-cell-autonomously. The TNFR in DARE cells, Wengen, likely activated by ROS, strongly promotes DARE proliferation, while TNF/Eiger and TNFR Grindelwald moderately suppress it. Downstream, p38 MAPK is the main signaling essential for DARE and NARE cell proliferation. Myo1D ensures DARE survival by preventing lethal effector caspase activation, whereas Myo7A/Crinkled supports moderate caspase activity. Dying cells trigger DARE induction, and both DARE and NARE transmit apoptosis resistance to progeny, with DARE progeny showing enhanced resistance. Maintaining balanced DARE-NARE proliferation is crucial for proper regeneration, growth, and differentiation, insights that may be relevant to radiation-resistant cells in cancer therapy.
-
(2025) Nature Structural and Molecular Biology. 32, 10, p. 1854-1858 Abstract[All authors]
Cell death contributes to tissue homeostasis and plays critical roles in inflammation and host defense. Our increasing understanding of the physiological importance of cell death underlines the need to more fully elucidate its underlying mechanisms in health and disease. Molecular and structural insight into the cell death apparatus could provide strategies to target the loss of cells in pathophysiological contexts. We asked experts studying a range of cell death types to share with us what they are most excited to tackle and what the field needs for progress.
-
(2024) Nature Communications. 15, 5715. Abstract[All authors]
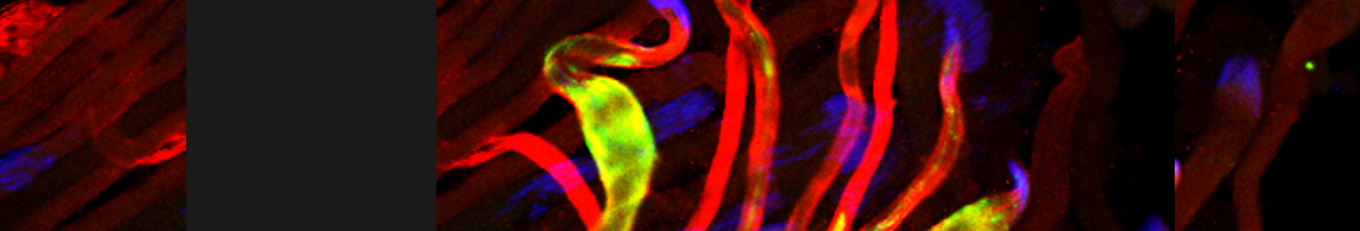
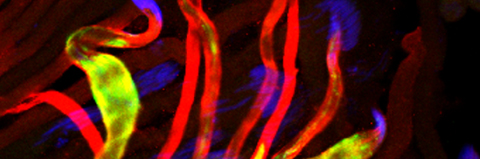
Mitochondria are maternally inherited, but the mechanisms underlying paternal mitochondrial elimination after fertilization are far less clear. Using Drosophila, we show that special egg-derived multivesicular body vesicles promote paternal mitochondrial elimination by activating an LC3-associated phagocytosis-like pathway, a cellular defense pathway commonly employed against invading microbes. Upon fertilization, these egg-derived vesicles form extended vesicular sheaths around the sperm flagellum, promoting degradation of the sperm mitochondrial derivative and plasma membrane. LC3-associated phagocytosis cascade of events, including recruitment of a Rubicon-based class III PI(3)K complex to the flagellum vesicular sheaths, its activation, and consequent recruitment of Atg8/LC3, are all required for paternal mitochondrial elimination. Finally, lysosomes fuse with strings of large vesicles derived from the flagellum vesicular sheaths and contain degrading fragments of the paternal mitochondrial derivative. Given reports showing that in some mammals, the paternal mitochondria are also decorated with Atg8/LC3 and surrounded by multivesicular bodies upon fertilization, our findings suggest that a similar pathway also mediates paternal mitochondrial elimination in other flagellated sperm-producing organisms.
-
(2023) HGG advances. 4, 3, 100189. Abstract[All authors]
Quantitative and qualitative spermatogenic impairments are major causes of mens infertility. Although in vitro fertilization (IVF) is effective, some couples persistently fail to conceive. To identify causal variants in patients with severe male infertility factor and repeated IVF failures, we sequenced the exome of two consanguineous family members who underwent several failed IVF cycles and were diagnosed with low sperm count and motility. We identified a rare homozygous nonsense mutation in a previously uncharacterized gene, RNF212B, as the causative variant. Recurrence was identified in another unrelated, infertile patient who also faced repeated failed IVF treatments. scRNA-seq demonstrated meiosis-specific expression of RNF212B. Sequence analysis located a protein domain known to be associated with aneuploidy, which can explain multiple IVF failures. Accordingly, FISH analysis revealed a high aneuploidy rate in the patients' sperm cells and their IVF embryos. Finally, inactivation of the Drosophila orthologs significantly reduced male fertility. Given that members of the evolutionary conserved RNF212 gene family are involved in meiotic recombination and crossover maturation, our findings indicate a critical role of RNF212B in meiosis, genome stability, and in human fertility. Since recombination is completely absent in Drosophila males, our findings may indicate an additional unrelated role for the RNF212-like paralogs in spermatogenesis. We report the discovery of a pathogenic variant in the uncharacterized human gene RNF212B, which belongs to an evolutionary conserved gene family involved in meiotic recombination. RNF212B loss of function is associated with sperm abnormalities and broad aneuploidy in patient-derived sperm cells and embryos, resulting in multiple repeated IVF failures.
-
(2023) Cell Death and Differentiation. 30, 5, p. 1097-1154 Abstract[All authors]
Apoptosis is a form of regulated cell death (RCD) that involves proteases of the caspase family. Pharmacological and genetic strategies that experimentally inhibit or delay apoptosis in mammalian systems have elucidated the key contribution of this process not only to (post-)embryonic development and adult tissue homeostasis, but also to the etiology of multiple human disorders. Consistent with this notion, while defects in the molecular machinery for apoptotic cell death impair organismal development and promote oncogenesis, the unwarranted activation of apoptosis promotes cell loss and tissue damage in the context of various neurological, cardiovascular, renal, hepatic, infectious, neoplastic and inflammatory conditions. Here, the Nomenclature Committee on Cell Death (NCCD) gathered to critically summarize an abundant pre-clinical literature mechanistically linking the core apoptotic apparatus to organismal homeostasis in the context of disease.
-
(2021) Nature Communications. 12, 1, 2285. Abstract
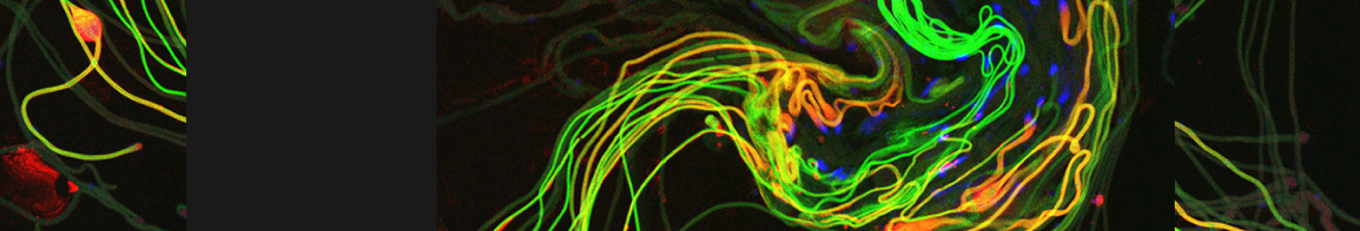
During Drosophila embryonic development, cell death eliminates 30% of the primordial germ cells (PGCs). Inhibiting apoptosis does not prevent PGC death, suggesting a divergence from the conventional apoptotic program. Here, we demonstrate that PGCs normally activate an intrinsic alternative cell death (ACD) pathway mediated by DNase II release from lysosomes, leading to nuclear translocation and subsequent DNA double-strand breaks (DSBs). DSBs activate the DNA damage-sensing enzyme, Poly(ADP-ribose) (PAR) polymerase-1 (PARP-1) and the ATR/Chk1 branch of the DNA damage response. PARP-1 and DNase II engage in a positive feedback amplification loop mediated by the release of PAR polymers from the nucleus and the nuclear accumulation of DNase II in an AIF- and CypA-dependent manner, ultimately resulting in PGC death. Given the anatomical and molecular similarities with an ACD pathway called parthanatos, these findings reveal a parthanatos-like cell death pathway active during Drosophila development.
-
(2021) FEBS Journal. 288, 22, p. 6310-6314 Abstract
In this special interview series, we profile members of The FEBS Journal editorial board to highlight their research focus, perspectives on the journal and future directions in their field. Eli Arama is an Associate Professor at the Weizmann Institute of Science in Rehovot, Israel. He has served as an editorial board member of The FEBS Journal since 2018.
-
(2020) FEBS Journal. 288, 7, p. 2166-2183 Abstract
Apoptosis is a major form of programmed cell death (PCD) that eliminates unnecessary and potentially dangerous cells in all metazoan organisms, thus ensuring tissue homeostasis and many developmental processes. Accordingly, defects in the activation of the apoptotic pathway often pave the way to disease. After several decades of intensive research, the molecular details controlling the apoptosis program have largely been unraveled, as well as the regulatory mechanisms of caspase activation during apoptosis. Nevertheless, an ever-growing list of studies is suggesting the essential role of caspases and other apoptotic proteins in ensuring nonlethal cellular functions during normal development, tissue repair, and regeneration. Moreover, if deregulated, these novel nonapoptotic functions can also instigate diseases. The difficulty of identifying and manipulating the caspase-dependent nonlethal cellular processes (CDPs), as well as the nonlethal functions of other cell death proteins (NLF-CDPs), meant that CDPs and NLF-CDPs have been only curiosities within the apoptotic field; however, the recent technical advancements and the latest biological findings are assigning an unanticipated biological significance to these nonapoptotic functions. Here, we summarize the various talks presented in the first international conference fully dedicated to discuss CDPs and NFL-CDPs and named The Batsheva de Rothschild Seminar on Non-Apoptotic Roles of Apoptotic Proteins. The conference was organized between September 22, 2019, and 25, 2019, by Eli Arama (Weizmann Institute of Science), Luis Alberto Baena-Lopez (University of Oxford), and Howard O. Fearnhead (NUI Galway) at the Weizmann Institute of Science in Israel, and hosted a large international group of researchers.
-
(2019) Human Reproduction. 34, 4, p. 666-671 Abstract
STUDY QUESTION Are there genetic variants that can be used for the clinical evaluation of azoospermic men?SUMMARY ANSWER A novel homozygous frame-shift mutation in the MEIOB gene was identified in three azoospermic patients from two different families.WHAT IS KNOWN ALREADY Up to 1% of all men have complete absence of sperm in the semen, a condition known as azoospermia. There are very few tools for determining the etiology of azoospermia and the likelihood of sperm cells in the testis. The MEIOB gene codes for a single-strand DNA binding protein required for DNA double-strand breaks repair during meiosis. MEIOB appears to be exclusively expressed in human and mouse testis, and MeioB knockout mice are azoospermic due to meiotic arrest.STUDY DESIGN, SIZE, DURATION Two brothers with non-obstructive azoospermia (NOA) underwent whole-exome sequencing followed by comprehensive bioinformatics analyses. Candidate variations were further screened in infertile and fertile men, as well as in public and local reference databases.PARTICIPANTS/MATERIALS, SETTING, METHODS This study included 159 infertile and 77 fertile men. The exomes of two Arab men were completely sequenced. In addition, 213 other men of the same Arab ethnicity (136 infertile and 77 fertile men) underwent restriction fragment length polymorphism (RFLP) screening, as did 21 NOA men, of other ethnicities, with testicular impairment of spermatocyte arrest. All of the infertile men underwent Y-chromosome microdeletion and CFTR gene mutation assessments. Comprehensive bioinformatics analyses were designed to uncover candidate mutations associated with azoospermia.MAIN RESULTS AND THE ROLE OF CHANCE A novel homozygous frame-shift mutation in the MEIOB gene was identified in two brothers of Arab ethnicity. This frame-shift is predicted to result in a truncated MEIOB protein, which lacks the conserved C-terminal DNA binding domain. RFLP screening of the mutation in 157 infertile men, including 112 NOA patients of Arab ethnicity, identified an additional unrelated NOA patient with the same homozygous mutation and a similar testicular impairment. This mutation was not found in available public databases (n > 160 000), nor in the 77 proven fertile men, nor in our database of local Israeli population variations derived from exome and genome sequencing data (n = 500).LIMITATIONS, REASONS FOR CAUTION We have thus far screened for only two specific MEIOB probable pathogenic mutations in a relatively small local cohort. Therefore, the relative incidence of MEIOB mutations in azoospermia should be further assessed in larger and diverse cohorts in order to determine the efficiency of MEIOB sequence screening for clinical evaluations.WIDER IMPLICATIONS OF THE FINDINGS The relatively high incidence of likely NOA-causing mutations in MEIOB that was found in our cohort supports the idea that a complete screening of this gene might be beneficial for clinical evaluation of NOA patients.STUDY FUNDING/COMPETING INTEREST(S) This research was supported in part by a grant to EA from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC grant agreement (616088). There are no competing interests.TRIAL REGISTRATION NUMBER N/A.
-
(2018) Nature Communications. 9, 1, 2806. Abstract
Maintenance of tissue integrity during development and homeostasis requires the precise coordination of several cell-based processes, including cell death. In animals, the majority of such cell death occurs by apoptosis, a process mediated by caspase proteases. To elucidate the role of caspases in tissue integrity, we investigated the behavior of Drosophila epithelial cells that are severely compromised for caspase activity. We show that these cells acquire migratory and invasive capacities, either within 1-2 days following irradiation or spontaneously during development. Importantly, low levels of effector caspase activity, which are far below the threshold required to induce apoptosis, can potently inhibit this process, as well as a distinct, developmental paradigm of primordial germ cell migration. These findings may have implications for radiation therapy in cancer treatment. Furthermore, given the presence of caspases throughout metazoa, our results could imply that preventing unwanted cell migration constitutes an ancient non-apoptotic function of these proteases.
-
(2018) Cell Communication and Signaling. 16, 1, 34. Abstract
The International Conference on Cell Death in Cancer and Toxicology 2018 (February 20-22, 2018) provided an international forum for scientific collaborations across multiple disciplines in cancer, cell death, and toxicology. During the three-day symposium, researchers and clinicians shared recent advances in basic, clinical, and translational research in cancer. Several student poster abstracts were selected for platform talks and many young investigators participated in the meeting. Together, this highly interactive meeting showcased the rapid expansion in biomedical research in India and paved the way for future meetings on cell death and cancer throughout India.
-
Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018(2018) Cell Death and Differentiation. 25, 3, p. 486-541 Abstract[All authors]
Over the past decade, the Nomenclature Committee on Cell Death (NCCD) has formulated guidelines for the definition and interpretation of cell death from morphological, biochemical, and functional perspectives. Since the field continues to expand and novel mechanisms that orchestrate multiple cell death pathways are unveiled, we propose an updated classification of cell death subroutines focusing on mechanistic and essential (as opposed to correlative and dispensable) aspects of the process. As we provide molecularly oriented definitions of terms including intrinsic apoptosis, extrinsic apoptosis, mitochondrial permeability transition (MPT)-driven necrosis, necroptosis, ferroptosis, pyroptosis, parthanatos, entotic cell death, NETotic cell death, lysosome-dependent cell death, autophagy-dependent cell death, immunogenic cell death, cellular senescence, and mitotic catastrophe, we discuss the utility of neologisms that refer to highly specialized instances of these processes. The mission of the NCCD is to provide a widely accepted nomenclature on cell death in support of the continued development of the field.
-
(2017) PLoS Genetics. 13, 9, e1007024. Abstract
The importance of regulated necrosis in pathologies such as cerebral stroke and myocardial infarction is now fully recognized. However, the physiological relevance of regulated necrosis remains unclear. Here, we report a conserved role for p53 in regulating necrosis in Drosophila and mammalian spermatogenesis. We found that Drosophila p53 is required for the programmed necrosis that occurs spontaneously in mitotic germ cells during spermatogenesis. This form of necrosis involved an atypical function of the initiator caspase Dronc/Caspase 9, independent of its catalytic activity. Prevention of p53-dependent necrosis resulted in testicular hyperplasia, which was reversed by restoring necrosis in spermatogonia. In mouse testes, p53 was required for heat-induced germ cell necrosis, indicating that regulation of necrosis is a primordial function of p53 conserved from invertebrates to vertebrates. Drosophila and mouse spermatogenesis will thus be useful models to identify inducers of necrosis to treat cancers that are refractory to apoptosis.
[All authors] -
-
(2017) PLoS Genetics. 13, 2, 1006545. Abstract
Every day, billions of human cells terminate their normal activities and launch intrinsic suicide pathways. Timely cell death is orchestrated by destructive functions encoded by dying cells, such as caspase proteases in the case of apoptotic or pyroptotic cell death. Caspases cleave specific intracellular substrates to kill and dismantle cells destined for elimination, and their dysregulation leads to a range of human disorders [1]. Drosophila models have proven to be key tools for understanding the regulation of caspases in cell death, but have also revealed other unanticipated roles for caspases, including cell proliferation [2], sperm maturation [3] and neuronal pruning [4]. Furthermore, the presence of widespread, nonlethal caspase activity in fly tissues suggests that there could be additional caspase-dependent processes besides those that are already known [5]. Thus, a new challenge is to understand the connections between non-death and pro-death functions of caspases. A new study from the Bergmann lab [6] reveals that mono-ubiquitylation of the Drosophila caspase Dronc inhibits apoptosis; more surprisingly, mono-ubiquitylation also inhibits an alternative role of Dronc. This work uncovered a role for Dronc in several non-lethal activities and organismal survival that apparently does not require Dronc's protease activity.
-
(2016) Cell Death Differ. 23, 12, p. 2019-2030 Abstract
De-ubiquitylating enzymes (DUBs) reverse protein ubiquitylation and thereby control essential cellular functions. Screening for a DUB that counteracts caspase ubiquitylation to regulate cell survival, we identified the Drosophila ovarian tumour-type DUB DUBA (CG6091). DUBA physically interacts with the initiator caspase death regulator Nedd2-like caspase (Dronc) and de-ubiquitylates it, thereby contributing to efficient inhibitor of apoptosis-antagonist-induced apoptosis in the fly eye. Searching also for non-apoptotic functions of DUBA, we found that Duba-null mutants are male sterile and display defects in spermatid individualisation, a process that depends on non-apoptotic caspase activity. Spermatids of DUBA-deficient flies showed reduced caspase activity and lack critical structures of the individualisation process. Biochemical characterisation revealed an obligate activation step of DUBA by phosphorylation. With genetic rescue experiments we demonstrate that DUBA phosphorylation and catalytic activity are crucial in vivo for DUBA function in spermatogenesis. Our results demonstrate for the first time the importance of de-ubiquitylation for fly spermatogenesis.
-
(2016) Developmental Cell. 37, 1, p. 15-33 Abstract
How cells avoid excessive caspase activity and unwanted cell death during apoptotic caspase-mediated removal of large cellular structures is poorly understood. We investigate caspase-mediated extrusion of spermatid cytoplasmic contents in Drosophila during spermatid individualization. We show that a Krebs cycle component, the ATP-specific form of the succinyl-CoA synthetase β subunit (A-Sβ), binds to and activates the Cullin-3-based ubiquitin ligase (CRL3) complex required for caspase activation in spermatids. In vitro and in vivo evidence suggests that this interaction occurs on the mitochondrial surface, thereby limiting the source of CRL3 complex activation to the vicinity of this organelle and reducing the potential rate of caspase activation by at least 60%. Domain swapping between A-Sβ and the GTP-specific SCSβ (G-Sβ), which functions redundantly in the Krebs cycle, show that the metabolic and structural roles of A-Sβ in spermatids can be uncoupled, highlighting a moonlighting function of this Krebs cycle component in CRL activation.
-
(2016) Nature Communications. 7, 10972. Abstract[All authors]
Caspases provide vital links in non-apoptotic regulatory networks controlling inflammation, compensatory proliferation, morphology and cell migration. How caspases are activated under non-apoptotic conditions and process a selective set of substrates without killing the cell remain enigmatic. Here we find that the Drosophila unconventional myosin CRINKLED (CK) selectively interacts with the initiator caspase DRONC and regulates some of its non-apoptotic functions. Loss of CK in the arista, border cells or proneural clusters of the wing imaginal discs affects DRONC-dependent patterning. Our data indicate that CK acts as substrate adaptor, recruiting SHAGGY46/GSK3-β to DRONC, thereby facilitating caspase-mediated cleavage and localized modulation of kinase activity. Similarly, the mammalian CK counterpart, MYO7A, binds to and impinges on CASPASE-8, revealing a new regulatory axis affecting receptor interacting protein kinase-1 (RIPK1)>CASPASE-8 signalling. Together, our results expose a conserved role for unconventional myosins in transducing caspase-dependent regulation of kinases, allowing them to take part in specific signalling events.
-
-
Klionsky et al. (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 12(1):1-222.
-
(2014) Developmental Cell. 29, 3, p. 305-320 Abstract
Almost all animals contain mitochondria of maternal origin only, but the exact mechanisms underlying this phenomenon are still vague. We investigated the fate of Drosophila paternal mitochondria after fertilization. We demonstrate that the sperm mitochondrial derivative (MD) is rapidly eliminated in a stereotypical process dubbed paternal mitochondrial destruction (PMD). PMD is initiated by a network of vesicles resembling multivesicular bodies and displaying common features of the endocytic and autophagic pathways. These vesicles associate with the sperm tail and mediate the disintegration of its plasma membrane. Subsequently, the MD separates from the axoneme and breaks into smaller fragments, which are then sequestered by autophagosomes for degradation in lysosomes. We further provide evidence for the involvement of the ubiquitin pathway and the autophagy receptor p62 in this process. Finally, we show that the ubiquitin ligase Parkin is not involved in PMD, implying a divergence from the autophagic pathway of damaged mitochondria.
-
(2013) Developmental Cell. 25, 1, p. 29-42 Abstract
In both flies and mammals, almost one-third of the newly emerging male germ cells are spontaneously eliminated before entering meiosis. Here, we show that in Drosophila, germ cell death (GCD) involves the initiator caspase Dronc independently of the apoptosome and the main executioner caspases. Electron microscopy of dying germ cells revealed mixed morphologies of apoptosis and necrosis. We further show that the lysosomes and their catabolic enzymes, but not macroautophagy, are involved in the execution of GCD. We then identified, in a screen, the Parkinson's disease-associated mitochondrial protease, HtrA2/Omi, as an important mediator of GCD, acting mainly through its catalytic activity rather than by antagonizing inhibitor of apoptosis proteins. Concomitantly, other mitochondrial-associated factors were also implicated in GCD, including Pink1 (but not Parkin), the Bcl-2-related proteins, and endonuclease G, which establish the mitochondria as central mediators of GCD. These findings uncover an alternative developmental cell death pathway in metazoans.
-
(2012) Developmental Cell. 23, 1, p. 5-6 Abstract
What are the origins of programmed cell death (PCD)? In this issue of Developmental Cell, Eastwood et al. (2012) uncover an ancient developmental program of nuclear destruction in yeast, implying that some PCD mechanisms could have emerged from nonlethal processes before the divergence of fungi and metazoan.
-
(2012) Journal of Cell Biology. 196, 4, p. 513-527 Abstract
Essentially, all metazoan cells can undergo apoptosis, but some cells are more sensitive than others to apoptotic stimuli. To date, it is unclear what determines the apoptotic potential of the cell. We set up an in vivo system for monitoring and comparing the activity levels of the two main effector caspases in Drosophila melanogaster, Drice and Dcp-1. Both caspases were activated by the apoptosome after irradiation. However, whereas each caspase alone could induce apoptosis, Drice was a more effective inducer of apoptosis than Dcp-1, which instead had a role in establishing the rate of cell death. These functional differences are attributed to their intrinsic properties rather than merely their tissue specificities. Significantly, the levels of the procaspases are directly proportional to their activity levels and play a key role in determining the cell's sensitivity to apoptosis. Finally, we provide evidence for the existence of a cellular execution threshold of caspase activity, which must be reached to induce apoptosis.
-
(2011) Developmental Cell. 20, 5, p. 575-576 Abstract
Members of the Bcl-2 family proteins are best known for their roles in apoptosis regulation. In this issue of Developmental Cell, Popgeorgiev et al. (2011) have uncovered a new, nonapoptotic role for a Bcl-2 homolog during early embryogenesis in zebrafish.
-
(2011) Journal of Biological Chemistry. 286, 17, p. 15556-15564 Abstract
Apoptosis operates to eliminate damaged or potentially dangerous cells. This loss is often compensated by extra proliferation of neighboring cells. Studies in Drosophila imaginal discs suggest that the signal for the additional growth emanates from the dying cells. In particular, it was suggested that the initiator caspase Dronc mediates compensatory proliferation (CP) through Dp53inwing discs. However, the exact mechanism that governs this CP remained poorly understood. We have previously shown that elimination of misspecified cells due to reduced Dpp signaling is achieved by the interaction of the corepressor NAB with the transcriptional repressor Brk, which in turn induces Jun N-terminal kinase-dependent apoptosis. Here, we performed a systematic in vivo loss-and gain-of-function analysistostudy NAB-induced death and CP. Our findings indicate that the NAB primary signal activates JNK, which in turn transmits two independent signals. One triggers apoptosis through the pro-apoptotic proteins Reaper and Hid, which in turn promote activation of caspases by the apoptosome components Ark and Dronc. The other signal induces CP in a manner that is independent of the death signal, Dronc, or Dp53. Once induced, the apoptotic pathway further activates a CP response. Our data suggest that JNK is the candidate factor that differentiates between apoptosis that involves CP and apoptosis that does not.
-
(2010) Developmental Cell. 19, 1, p. 160-173 Abstract
Caspases are executioners of apoptosis but also participate in a variety of vital cellular processes. Here, we identified Soti, an inhibitor of the Cullin-3-based E3 ubiquitin ligase complex required for caspase activation during Drosophila spermatid terminal differentiation (individualization). We further provide evidence that the giant inhibitor of apoptosis-like protein dBruce is a target for the Cullin-3-based complex, and that Soti competes with dBruce for binding to Klhl10, the E3 substrate recruitment subunit. We then demonstrate that Soti is expressed in a subcellular gradient within spermatids and in turn promotes proper formation of a similar dBruce gradient. Consequently, caspase activation occurs in an inverse graded fashion, such that the regions of the developing spermatid that are the last to individualize experience the lowest levels of activated caspases. These findings elucidate how the spatial regulation of caspase activation can permit caspase-dependent differentiation while preventing full-blown apoptosis.
-
(2010) Journal of Neuroscience. 30, 18, p. 6375-6386 Abstract
Selective degeneration of neuronal projections and neurite pruning are critical for establishment and maintenance of functional neural circuits in both insects and mammals. However, the molecular mechanisms that govern developmental neurite pruning versus injury-induced neurite degeneration are still mostly unclear. Here, we show that the effector caspases 6 and 3 are both expressed within axons and that, on trophic deprivation, they exhibit distinct modes of activation. Surprisingly, inhibition of caspases is not sufficient for axonal protection and a parallel modulation of a NAD+-sensitive pathway is required. The proapoptotic protein BAX is a key element in both pathways as its genetic ablation protected sensory axons against developmental degeneration both in vitro and in vivo. Last, we demonstrate that both pathways are also involved in developmental dendritic pruning in Drosophila. More specifically, the mouse WldS (Wallerian degeneration slow) protein, which is mainly composed of the full-length sequence of the NAD+ biosynthetic Nmnat1 enzyme, can suppress dendritic pruning in C4da (class IV dendritic arborization) sensory neurons in parallel to the fly effector caspases. These findings indicate that two distinct autodestruction pathways act separately or in concert to regulate developmental neurite pruning.
-
(2010) Development. 137, 10, p. 1679-1688 Abstract
Terminal differentiation of male germ cells in Drosophila and mammals requires extensive cytoarchitectural remodeling, the elimination of many organelles, and a large reduction in cell volume. The associated process, termed spermatid individualization, is facilitated by the apoptotic machinery, including caspases, but does not result in cell death. From a screen for genes defective in caspase activation in this system, we isolated a novel F-box protein, which we termed Nutcracker, that is strictly required for caspase activation and sperm differentiation. Nutcracker interacts through its F-box domain with members of a Cullin-1-based ubiquitin ligase complex (SCF): Cullin-1 and SkpA. This ubiquitin ligase does not regulate the stability of the caspase inhibitors DIAP1 and DIAP2, but physically binds Bruce, a BIR-containing giant protein involved in apoptosis regulation. Furthermore, nutcracker mutants disrupt proteasome activity without affecting their distribution. These findings define a new SCF complex required for caspase activation during sperm differentiation and highlight the role of regulated proteolysis during this process.
-
(2010). A novel F-box protein is required for caspase activation during cellular remodeling in Drosophila. Development. 137:1679-88.
-
(2009) Apoptosis. 14, 8, p. 980-995 Abstract
Since the pioneering discovery that the genetic cell death program in C. elegans is executed by the cysteine-aspartate protease (caspase) CED3, caspase activation has become nearly synonymous with apoptosis. A critical mass of data accumulated in the past few years, have clearly established that apoptotic caspases can also participate in a variety of non-apoptotic processes. The roles of caspases during these processes and the regulatory mechanisms that prevent unrestrained caspase activity remain to be fully investigated, and may vary in different cellular contexts. Significantly, some of these processes, such as terminal differentiation of vertebrate lens fiber cells and red blood cells, as well as spermatid terminal differentiation and dendritic pruning of sensory neurons in Drosophila, all involve proteolytic degradation of major cellular compartments, and are conceptually, molecularly, biochemically, and morphologically reminiscent of apoptosis. Moreover, some of these model systems bear added values for the study of caspase activation/apoptosis. For example, the Drosophila sperm differentiation is the only system known in invertebrate which absolutely requires the mitochondrial pathway (i.e. Cyt c). The existence of testis-specific genes for many of the components in the electron transport chain, including Cyt c, facilitates the use of the Drosophila sperm system to investigate possible roles of these otherwise essential proteins in caspase activation. Caspases are also involved in a wide range of other vital processes of non-degenerative nature, indicating that these proteases play much more diverse roles than previously assumed. In this essay, we review genetic, cytological, and molecular studies conducted in Drosophila, vertebrate, and cultured cells, which underlie the foundations of this newly emerging field.
-
(2009) Journal of Cell Science. 122, 4, p. 471-480 Abstract
Endocytosis, which is a key process in eukaryotic cells, has a central role in maintaining cellular homeostasis, nutrient uptake, development and downregulation of signal transduction. This complex process depends on several protein-protein interactions mediated by specific modules. One such module is the EH domain. The EH-domain-containing proteins comprise a family that includes four vertebrate members (EHD1-EHD4) and one Drosophila ortholog, Past1. We used Drosophila as a model to understand the physiological role of this family of proteins. We observed that the two predicted Past1 transcripts are differentially expressed both temporally and spatially during the life cycle of the fly. Endogenous Past1 as well as Past1A and Past1B, expressed from plasmids, were localized mainly to the membrane of Drosophila-derived cells. We generated mutants in the Past1 gene by excising a P-element inserted in it. The Past1 mutants reached adulthood but died precociously. They were temperature sensitive and infertile because of lesions in the reproductive system. Garland cells that originated from Past1 mutants exhibited a marked decrease in their ability to endocytose fluorescently labeled avidin. Genetic interaction was found between Past1 and members of the Notch signaling pathway, suggesting a role for Past1 in this developmentally crucial signaling pathway.
-
-
-
(2007) PLoS Biology. 5, 10, p. 2270-2287 Abstract
In both insects and mammals, spermatids eliminate their bulk cytoplasm as they undergo terminal differentiation. In Drosophila, this process of dramatic cellular remodeling requires apoptotic proteins, including caspases. To gain further insight into the regulation of caspases, we screened a large collection of sterile male flies for mutants that block effector caspase activation at the onset of spermatid individualization. Here, we describe the identification and characterization of a testis-specific, Cullin-3 -dependent ubiquitin ligase complex that is required for caspase activation in spermatids. Mutations in either a testis-specific isoform of Cullin-3 (Cul3(Testis)), the small RING protein Roc1b, or a Drosophila orthologue of the mammalian BTB-Kelch protein Klhl10 all reduce or eliminate effector caspase activation in spermatids. Importantly, all three genes encode proteins that can physically interact to form a ubiquitin ligase complex. Roc1b binds to the catalytic core of Cullin-3, and Klhl10 binds specifically to a unique testis-specific N-terminal Cullin-3 (TeNC) domain of Cul3Testis that is required for activation of effector caspase in spermatids. Finally, the BIR domain region of the giant inhibitor of apoptosis -like protein dBruce is sufficient to bind to Klhl10, which is consistent with the idea that dBruce is a substrate for the Cullin-3-based E3-ligase complex. These findings reveal a novel role of Cullin-based ubiquitin ligases in caspase regulation.
-
(2007) Cell Death and Differentiation. 14, 8, p. 1508-1517 Abstract
Programmed cell death (PCD) in the Drosophila ovary occurs either during mid- oogenesis, resulting in degeneration of the entire egg chamber or during late oogenesis, to facilitate the development of the oocyte. PCD during oogenesis is regulated by mechanisms different from those that control cell death in other Drosophila tissues. We have analyzed the role of caspases in PCD of the female germline by examining caspase mutants and overexpressing caspase inhibitors. Imprecise P- element excision was used to generate mutants of the initiator caspase strica. While null mutants of strica or another initiator caspase, dronc, display no ovary phenotype, we find that strica exhibits redundancy with dronc, during both mid- and late oogenesis. Ovaries of double mutants contain defective mid- stage egg chambers similar to those reported previously in dcp-1 mutants, and mature egg chambers with persisting nurse cell nuclei. In addition, the effector caspases drice and dcp-1 also display redundant functions during late oogenesis, resulting in persisting nurse cell nuclei. These findings indicate that caspases are required for nurse cell death during mid-oogenesis, and participate in developmental nurse cell death during late oogenesis. This reveals a novel pathway of cell death in the ovary that utilizes strica, dronc, dcp-1 and drice, and importantly illustrates strong redundancy among the caspases.
-
(2007). The Drosophila caspases Strica and Dronc function redundantly in programmed cell death during oogenesis. Cell Death Differ. 14:1507-1517.
-
(2006) EMBO Reports. 7, 9, p. 933-939 Abstract
The role of cytochrome c ( Cyt c) in caspase activation has largely been established from mammalian cell- culture studies, but much remains to be learned about its physiological relevance in situ. The role of Cyt c in invertebrates has been subject to considerable controversy. The Drosophila genome contains distinct cyt c genes: cyt c-p and cyt c-d. Loss of cyt c- p function causes embryonic lethality owing to a requirement of the gene for mitochondrial respiration. By contrast, cyt c- d mutants are viable but male sterile. Here, we show that cyt c- d regulates developmental apoptosis in the pupal eye. cyt c- d mutant retinas show a profound delay in the apoptosis of superfluous interommatidial cells and perimeter ommatidial cells. Furthermore, there is no apoptosis in mutant retinal tissues for the Drosophila homologues of apoptotic protease- activating factor 1 ( Ark) and caspase 9 ( Dronc). In addition, we found that cyt c- d - as with ark and dronc - regulates scutellar bristle number, which is known to depend on caspase activity. Collectively, our results indicate a role of Cyt c in caspase regulation of Drosophila somatic cells.
-
(2006) Nature Protocols. 1, 4, p. 1725-1731 Abstract
In Drosophila, vast numbers of cells undergo apoptosis during normal development. In addition, excessive apoptosis can be induced in response to a variety of stress or injury paradigms, including DNA damage, oxidative stress, nutrient deprivation, unfolded proteins and mechanical tissue damage. Two of the most commonly used methods to label apoptotic cells in Drosophila are terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) for fixed tissues and acridine orange (AO) staining for live embryos or tissues. Here, we describe protocols for labeling apoptotic cells in Drosophila embryos and adult male gonads. Slightly modified protocols can also be applied for other Drosophila tissues. The AO protocol is quick, simple and allows real-time imaging of doomed cells in live tissues. However, it is difficult to combine with conventional counterstains or Ab labeling. On the other hand, this functionality is readily afforded by the TUNEL protocol, which permits the detection of apoptotic cells in fixed tissues. These staining procedures can be completed in 1-2 d.
-
(2006) EMBO Journal. 25, 1, p. 232-243 Abstract
Cytochrome C has two apparently separable cellular functions: respiration and caspase activation during apoptosis. While a role of the mitochondria and cytochrome C in the assembly of the apoptosome and caspase activation has been established for mammalian cells, the existence of a comparable function for cytochrome C in invertebrates remains controversial. Drosophila possesses two cytochrome c genes, cyt-c-d and cyt-c-p. We show that only cyt-c-d is required for caspase activation in an apoptosis-like process during spermatid differentiation, whereas cyt-c-p is required for respiration in the soma. However, both cytochrome C proteins can function interchangeably in respiration and caspase activation, and the difference in their genetic requirements can be attributed to differential expression in the soma and testes. Furthermore, orthologues of the apoptosome components, Ark (Apaf-1) and Dronc (caspase-9), are also required for the proper removal of bulk cytoplasm during spermatogenesis. Finally, several mutants that block caspase activation during spermatogenesis were isolated in a genetic screen, including mutants with defects in spermatid mitochondrial organization. These observations establish a role for the mitochondria in caspase activation during spermatogenesis.
-
(2006). The two Drosophila cytochrome C proteins can function in both respiration and caspase activation. The EMBO J. 25:232-243.
-
-
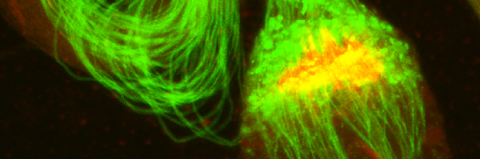
(2003) Developmental Cell. 4, 5, p. 687-697 Abstract
The final stage of spermatid terminal differentiation involves the removal of their bulk cytoplasm in a process known as spermatid individualization. Here we show that apoptotic proteins play an essential role during spermatid individualization in Drosophila melanogaster. Several aspects of sperm terminal differentiation, including the activation of caspases, are reminiscent of apoptosis. Notably, caspase inhibitors prevent the removal of bulk cytoplasm in spermatids and block sperm maturation in vivo, causing male sterility. We further identified loss-of-function mutations in one of the two Drosophila cyt-c genes, cyt-c-d, which block caspase activation and subsequent spermatid terminal differentiation. Finally, a giant ubiquitin-conjugating enzyme, dBruce, is required to protect the sperm nucleus against hypercondensation and degeneration. These observations suggest that an apoptosis-like mechanism is required for spermatid differentiation in Drosophila.