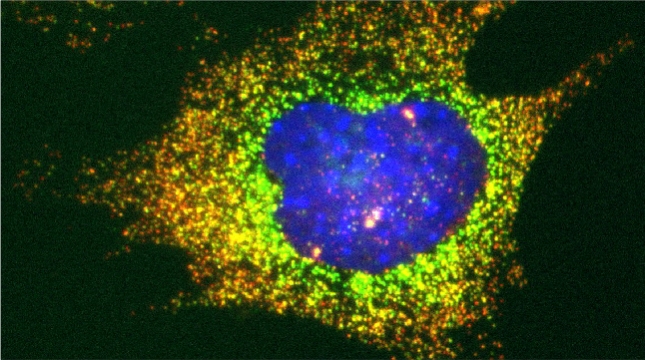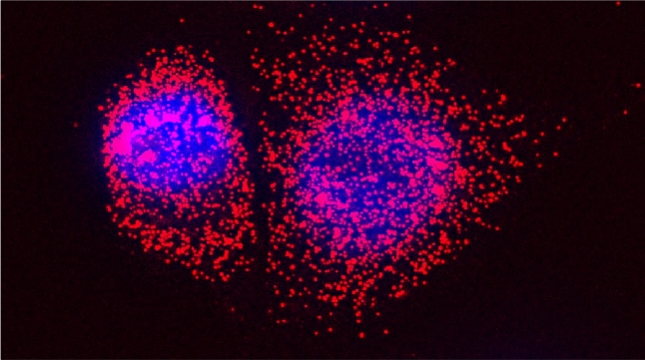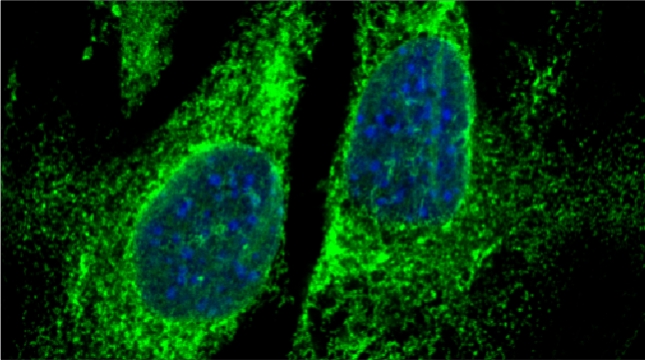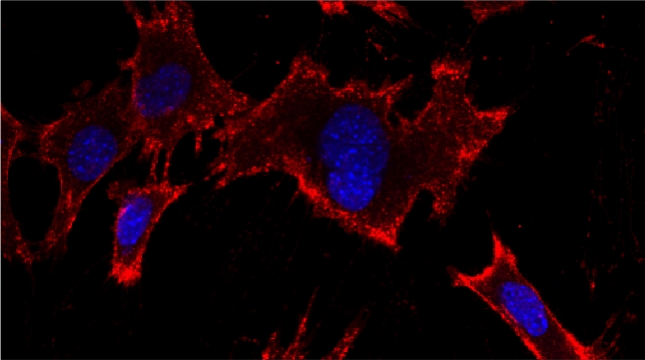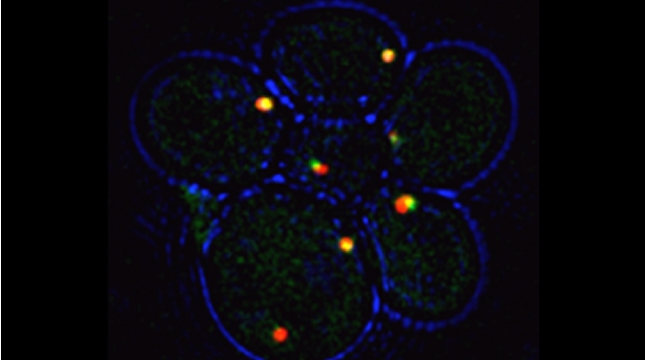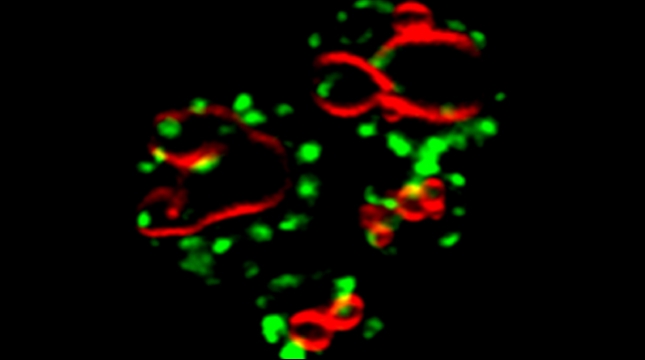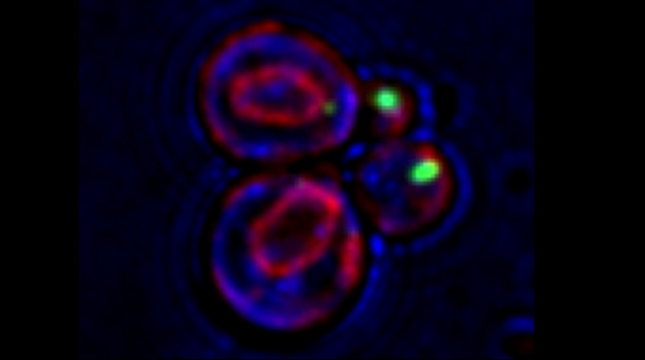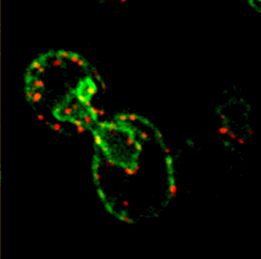
mRNAs encoding secreted and membrane proteins (mSMPs) were originally proposed to be recruited to the ER upon translation initiation as a part of an RNA-ribosome-nascent chain complex recognized by the signal recognition particle (SRP). However, it is becoming more and more apparent that cells use multiple means to import proteins into the ER via SRP-dependent and –independent mechanisms [Kraut-Cohen and Gerst, 2010]. Moreover, work from our lab using fluorescence microscopy and subcellular fractionation/RT-PCR has shown that a wide variety of mSMPs localize to ER membranes [Kraut-Cohen et al 2013]. Unlike mRNAs encoding polarity and secretion factors, which localize to cortical ER and are asymmetrically localized to polarized extensions in cells [Aronov et al 2007 and Gelin-Licht et al 2012], mSMPs localize to ER in mainly in the mother cell and are found in daughter cells after division. In addition, we show that mSMP localization to ER membranes is independent of both the SRP and translation initiation. Thus, mRNAs associate with the ER independently of translation. Ongoing work using RaPID [Slobodin and Gerst, 2010 and 2011] aims to identify the RNA-binding proteins necessary for mSMP localization to the ER. In addition, we have identified a novel cis-acting element present in RNAs encoding non-membranal secreted proteins. This element, called the SECReTE, plays a strong role in RNA localization to the ER and promoting protein secretion [Cohen-Zontag et al 2019]. We further found that SECReTE is enriched, and at least partially positionally conserved, in positive strand ssRNA viruses, such as coronaviruses, compared to negative strand ssRNA viruses [Haimovich et al 2020]. Our lab is currently working on dissecting the SECReTE sequence and on identifying the RNA binding protein which associates with SECReTE.