 The novel extended ex utero culture systems to grow mouse natural embryos through gastrulation and organogenesis that our group has recently developed, represent a long-awaited gold standard reference that will allow evaluating and improving the developmental potential of stem-cell-derived (synthetic) embryoid models generated by co-aggregating in vitro expanded stem cell lines. This line of research is conducted by utilizing in house developed electronic devices, advanced tissue culture capabilities, stem cell based cellular models combined with cutting-edge genomic editing, transgenics, advanced microscopy, optogenetics and single cell biology. We have shown that by starting solely with mouse naïve ESCs, we can unleash their ability to self-organize into embryoid models (termed SEMs or sEmbryos) that reach day 8.5 after completing gastrulation and initiating organogenesis. (Tarazi et al. Cell 2022).
The novel extended ex utero culture systems to grow mouse natural embryos through gastrulation and organogenesis that our group has recently developed, represent a long-awaited gold standard reference that will allow evaluating and improving the developmental potential of stem-cell-derived (synthetic) embryoid models generated by co-aggregating in vitro expanded stem cell lines. This line of research is conducted by utilizing in house developed electronic devices, advanced tissue culture capabilities, stem cell based cellular models combined with cutting-edge genomic editing, transgenics, advanced microscopy, optogenetics and single cell biology. We have shown that by starting solely with mouse naïve ESCs, we can unleash their ability to self-organize into embryoid models (termed SEMs or sEmbryos) that reach day 8.5 after completing gastrulation and initiating organogenesis. (Tarazi et al. Cell 2022).
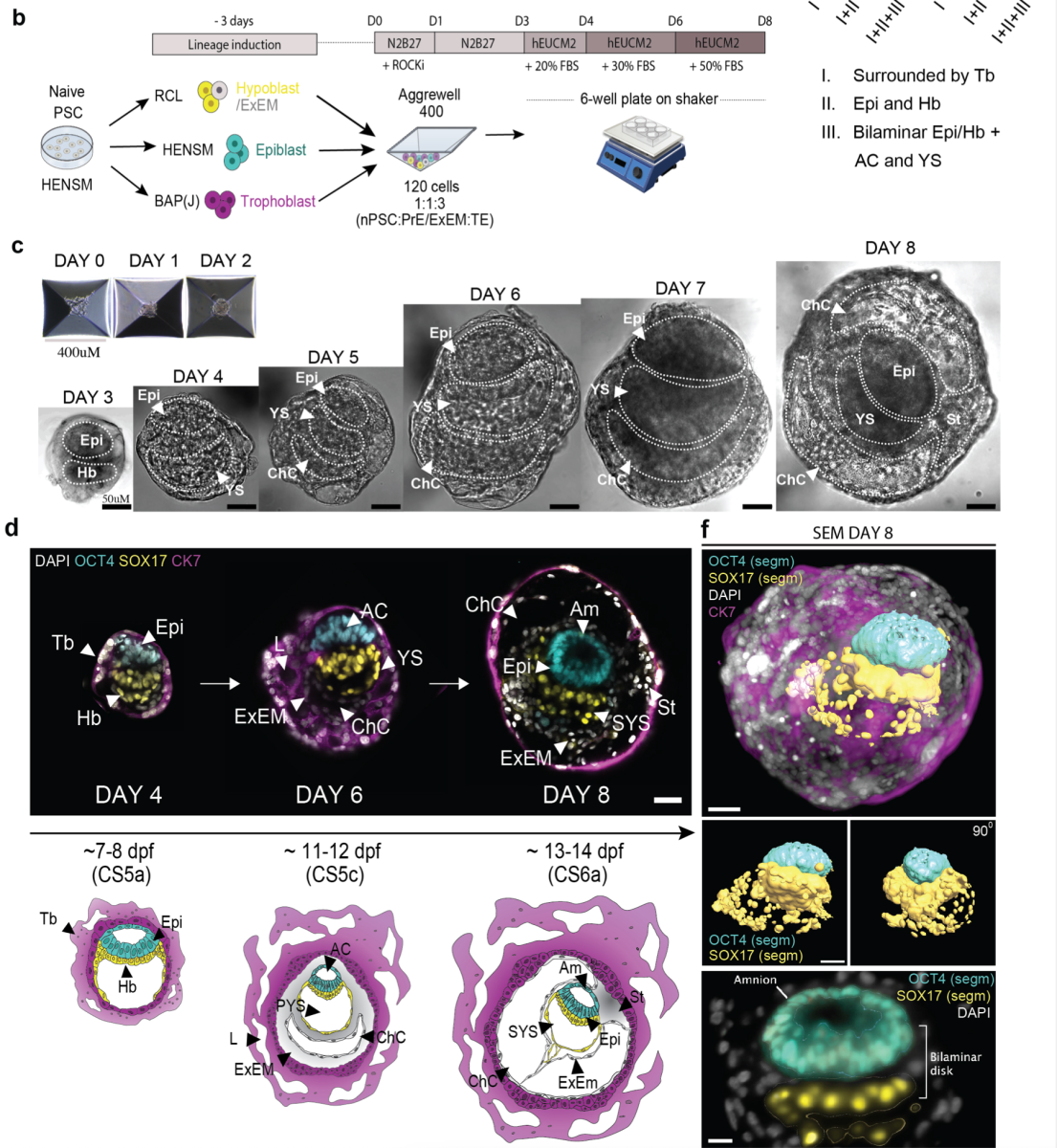
Recently we have also generated human SEMs, which model 14 days-post-fertilization embryo (Oldak et al. on BioRxiv). The model includes both embryonic and extra-embryonic (placental) tissues, and is of high interest for the research community, because this stage of human embryonic development (gastrulation and organogenesis), is typically not accessible for research.
This line of work may open a new path for developmental biology research and might establish universal platforms for generating early progenitor populations from iPSCs from a variety of species, via correctly inducing complex self-organization of stem cells in these unique artificial ex utero settings, that can be then used in stem cell differentiation research and bioengineering.
