Bacteria are social organisms that interact and coordinate their behaviors to shape our world. Whereas their clonal populations are genetically identical, they contain phenotypically distinct individuals. This diversity provides resilience to unpredictable environmental changes, such as antibiotic exposure or nutrient depletion. It also facilitates cooperative interactions between different sub-populations via specialization in costly activities such as virulence factor production and through metabolite exchanges, forming an extended basis for sociality. Yet, the phenotypic landscape in any given species remains largely unexplored due to the technical challenges of systematically profiling individual bacterial cells.
Our lab applies single-cell and spatial transcriptomics approaches to globally study cell-cell variation across lifestyles, from free-living populations and biofilms assemblies to bacterial colonization of host tissues. We are particularly interested in how single-cell heterogeneity manifests in plant-associated bacteria and opportunistic pathogens.
Relevant publications:
- Dar D, Dar N, Cai L, Newman DK. Spatial transcriptomics of planktonic and sessile bacterial populations at single-cell resolution. Science (2021)

Single bacterium transcriptional profiling with par-seqFISH. A two-stepped smFISH protocol allows for many genes to be measured sequentially in the same cells by selectively turning fluorescence ON or OFF. Each gene requires one fluorescent readout probe

Studying bacterial single-cell states directly within their native biofilm context via spatially resolved transcriptomics. Pseudomonas aeruginosa biofilms are shown in gray with par-seqFISH gene expression data in color. Biofilms in two developmental stages are shown on the left with a zoom-in (orange box) on the right. Cell boundaries are shown.
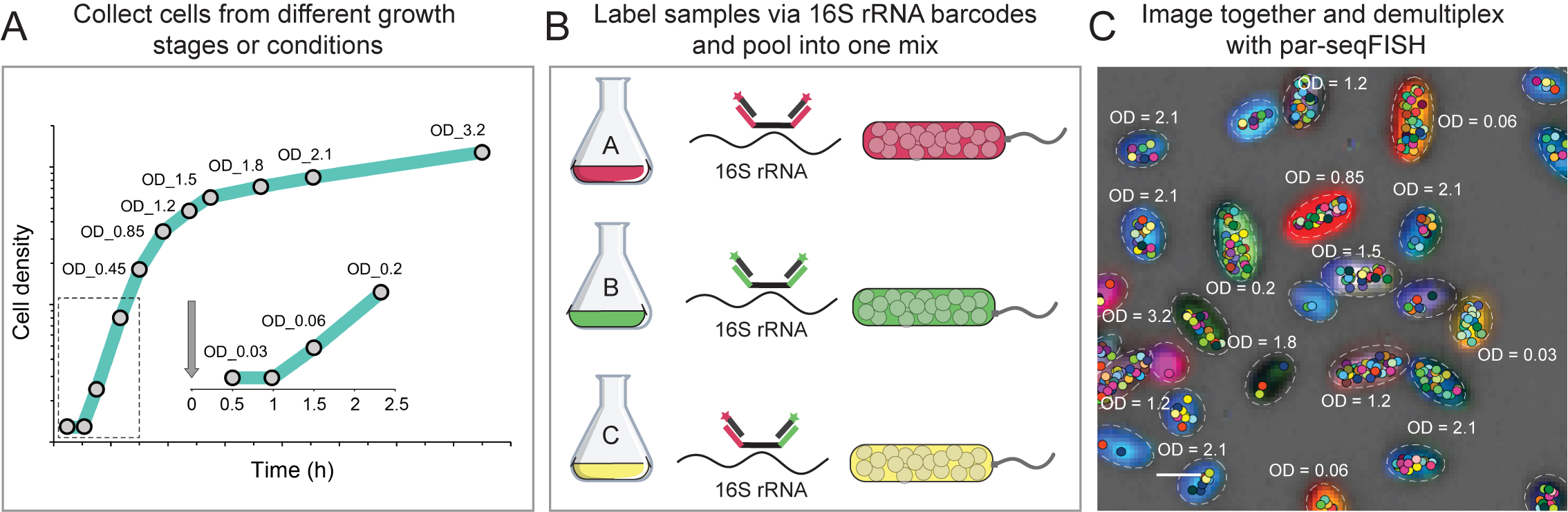
Analyzing dozens of growth conditions in parallel using Par-seqFISH. (A) Samples are collected at different time points. (B) Cells are labeled via their 16S-rRNA and pooled prior to expression analysis. (C) Cells are then imaged together and fluorescence reports the condition from which they were taken. Spots show the position of mRNAs with the cell.


