Crystal structures of cellulosomal modules
In order to understand the nature of the intermodular contacts formed between the cellulosomal subunits, or how polycellulosome assembly occurs, we employed crystallographic techniques. In early collaborative studies with Linda Shimon and Felix Frolow, we succeeded in determining the 3D crystal structure of a type-I cohesin. More recently, we successfully cloned, expressed, purified, crystallized and solved several novel structures of type-II and type-III cohesin modules from different cellulosomal species. A gallery of selected crystals grown in our lab is shown in Figure 1.
The type-II cohesin structure shares the same jelly-roll topology with the type-I cohesin structures, in which a flattened 9-stranded -sandwich is formed. However, several additional structural elements (an -helix and two “-flaps”) were observed that were lacking in the type-I structures (Figure 2A) (Noach et. al., 2003, Noach et. al., 2005, Noach et. al., 2008). These structural differences have been suggested to play a role in the type-specificity observed between the type-I and type-II cohesin-dockerin interactions.
The crystal structure of a type-III anchoring cohesin module from R. flavefaciens also revealed additional composite structural elements that represent a dramatic divergence from the other known cohesin structures (Figure 2B) (Alber et. al., 2008, Alber et. al., 2009). In this context, an elaborate -helix is located between -strands 8 and 9. The -helix is enveloped by an extensive N-terminal loop, unseen in any other known cohesin, which embraces the helix, thereby enhancing its stability.
The structure of a different type-III “adaptor” cohesin (Figure 2C) exhibited the standard jelly-roll topology without additional secondary structures. Despite the similarity with the type-I cohesins, the type-III adaptor cohesin is characterized by differences in the lengths of the -strands and loops, which might reflect the differences in specificity that are inherent in its dockerin-recognition properties.
Additionally, the crystal structure of a bi-modular type-II cohesin dyad has revealed a novel arrangement of two adjacent cohesins on the scaffoldin subunit. The two cohesins are oriented in antiparallel fashion, yet their dockerin interacting surfaces (b-strands 8, 3, 6, 5) face the same direction — i.e., aligned on the same plane (Figure 2D). This work has been influenced greatly by close collaboration with Steven P. Smith, in which a “divide-and-build” approach has been taken for eventual determination of the crystal structure of a model scaffoldin and then a model cellulosome.
In an effort to understand the structural basis for assembly and cell-surface attachment of the cellulosome in R. flavefaciens, we determined the crystal structure of the high-affinity complex (Kd = 20.83 nM) between the ScaE cohesin module (CohE) and its cognate X-dockerin modular dyad (XDoc) from CttA at 1.97-Å resolution (Figure 3A)(Salama-Alber et. al., 2013). The structure reveals an atypical calcium-binding loop containing a 13-residue insert. The results further pinpoint two charged specificity-related residues on the surface of the cohesin module, which are responsible for specific vs. promiscuous cross-strain binding of the dockerin module. In addition, a combined functional role for the three enigmatic dockerin inserts was established (Figure 3B, 3C), whereby these extraneous segments serve as structural buttresses that reinforce the stalk-like conformation of the X-module, thus segregating its tethered complement of cellulosomal components from the cell surface. The novel structure of the CohE–XDoc complex sheds light on divergent dockerin structure and function and provides insight into the specificity features of the type-IIIe cohesin-dockerin interaction.
In several cases, the cohesin structures solved in our work represent precedent structures – the first of their class to be solved. The above structures provide new insight into the structural diversity and arrangement of the various cohesin types within the parent scaffoldin subunits. Further work in this direction will be performed to solve cohesin-dockerin heterodimers and higher order scaffoldin structures.
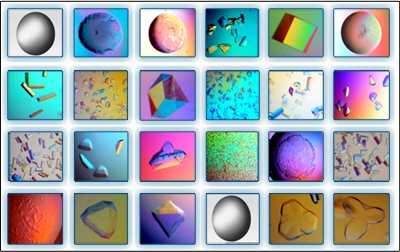
In order to understand the nature of the intermodular contacts formed between the cellulosomal subunits, or how polycellulosome assembly occurs, we employed crystallographic techniques. In early collaborative studies with Linda Shimon and Felix Frolow, we succeeded in determining the 3D crystal structure of a type-I cohesin. More recently, we successfully cloned, expressed, purified, crystallized and solved several novel structures of type-II and type-III cohesin modules from different cellulosomal species. A gallery of selected crystals grown in our lab is shown in Figure 1.
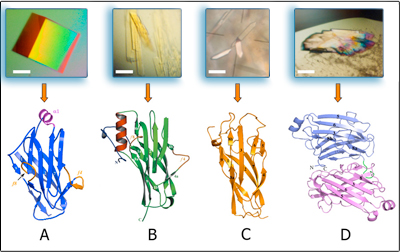
The crystal structure of a type-III anchoring cohesin module from R. flavefaciens also revealed additional composite structural elements that represent a dramatic divergence from the other known cohesin structures (Figure 2B) (Alber et. al., 2008, Alber et. al., 2009). In this context, an elaborate -helix is located between -strands 8 and 9. The -helix is enveloped by an extensive N-terminal loop, unseen in any other known cohesin, which embraces the helix, thereby enhancing its stability.
The structure of a different type-III “adaptor” cohesin (Figure 2C) exhibited the standard jelly-roll topology without additional secondary structures. Despite the similarity with the type-I cohesins, the type-III adaptor cohesin is characterized by differences in the lengths of the -strands and loops, which might reflect the differences in specificity that are inherent in its dockerin-recognition properties.
Additionally, the crystal structure of a bi-modular type-II cohesin dyad has revealed a novel arrangement of two adjacent cohesins on the scaffoldin subunit. The two cohesins are oriented in antiparallel fashion, yet their dockerin interacting surfaces (b-strands 8, 3, 6, 5) face the same direction — i.e., aligned on the same plane (Figure 2D). This work has been influenced greatly by close collaboration with Steven P. Smith, in which a “divide-and-build” approach has been taken for eventual determination of the crystal structure of a model scaffoldin and then a model cellulosome.
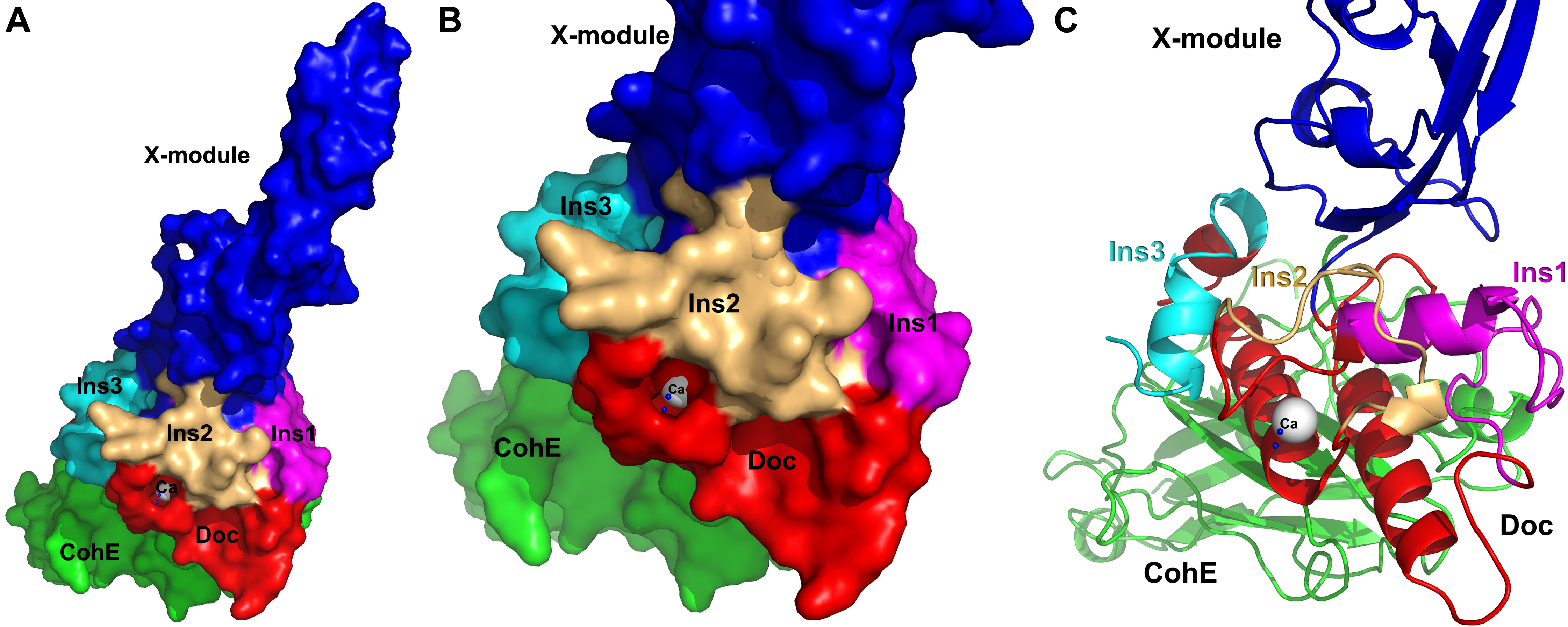
In an effort to understand the structural basis for assembly and cell-surface attachment of the cellulosome in R. flavefaciens, we determined the crystal structure of the high-affinity complex (Kd = 20.83 nM) between the ScaE cohesin module (CohE) and its cognate X-dockerin modular dyad (XDoc) from CttA at 1.97-Å resolution (Figure 3A)(Salama-Alber et. al., 2013). The structure reveals an atypical calcium-binding loop containing a 13-residue insert. The results further pinpoint two charged specificity-related residues on the surface of the cohesin module, which are responsible for specific vs. promiscuous cross-strain binding of the dockerin module. In addition, a combined functional role for the three enigmatic dockerin inserts was established (Figure 3B, 3C), whereby these extraneous segments serve as structural buttresses that reinforce the stalk-like conformation of the X-module, thus segregating its tethered complement of cellulosomal components from the cell surface. The novel structure of the CohE–XDoc complex sheds light on divergent dockerin structure and function and provides insight into the specificity features of the type-IIIe cohesin-dockerin interaction.



