The major direction of my early work in this field was devoted to the study of the avidin-biotin complex and its general use as a tool in the biological sciences. The main reason for interest in this system is two-fold: (a) The interaction between avidin (or streptavidin) and biotin exhibits the highest known affinity (Ka ~ 1015 M-1) between a ligand and a protein (1), and (b) the avidin-biotin system has become a universal tool in many biotechnological applications (Figure 1). We were the first to biotinylate various macromolecules, such as antibodies and lectins. We also designed and synthesized a variety of additional group-specific biotinylating reagents and developed many new applications of the avidin-biotin system (Figure 2).
The overall objectives of our work on the avidin-biotin system have included the following:
- To better understand the avidin molecule (and other biotin-binding proteins) and its interaction with biotin as a model high-affinity system
- To apply the avidin-biotin system in a variety of applications, and
- To "improve upon nature" by modifying the avidin and/or streptavidin molecule using chemical, enzymatic or recombinant means.
Our future plans in this area will concentrate on further structural, chemical, and genetic engineering studies on the molecular nature of the complexes formed between avidin (and streptavidin) and the two ligands — biotin and HABA and derivatives thereof. We continue to study the quaternary structures of avidin and streptavidin and their propensity to form tetramers (Figure 1). In derivative work, we will further previous approaches for bioengineering of the avidin molecule to "correct" for some of its molecular "blemishes" for improved commercial application.
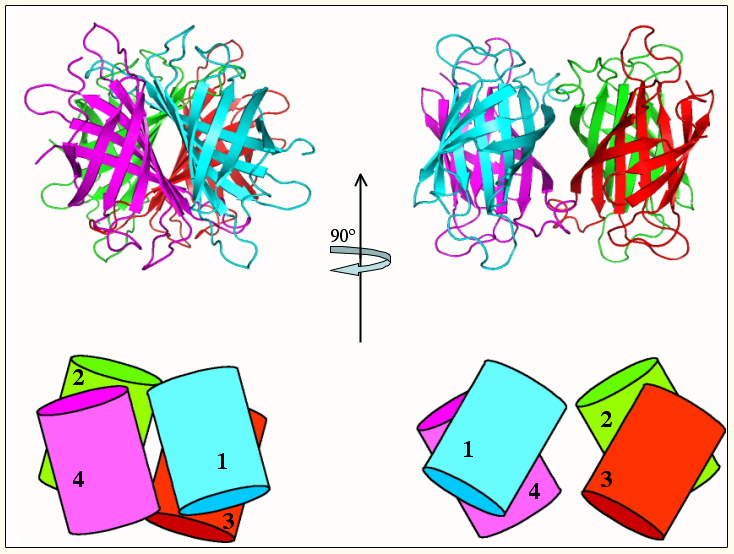
Figure 1: Schematic presentation of the general homotetrameric structure of the avidins. The ribbon diagram (top) is depicted from the avidin coordinates and the cartoon (bottom) represents the relative arrangement of the monomers (enumerated) in all three proteins. The tetramer at right is rotated clockwise by 90° along the vertical axis. The intimate interaction of the 1-4 (and 2-3) monomer-monomer interface is clearly visible in the figure at left. The contact surface of the alternative interface, comprising the dimer-dimer interaction (right), is much less extensive. Eisenberg-Domovich et al. Acta Crystallogr. (2005) D61, 528-38.
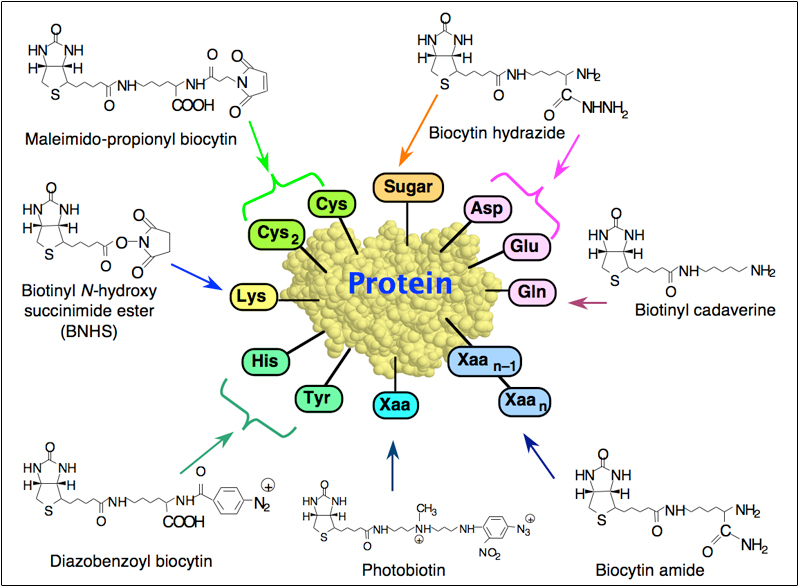
Figure 2. Some of the biotinylating reagents developed in our laboratory and the functional groups on proteins with which they interact.
References
Original Early Articles
Bayer, E. A., Viswanatha, T., and Wilchek, M. (1975) The use of a homologous series of affinity labeling reagents in the study of the biotin transport system in yeast cells. FEBS Lett. 60, 309-312.
Bayer, E. A., Skutelsky, E., Wynne, D., and Wilchek, M. (1976) The preparation of ferritin-avidin conjugates by reductive alkylation for use in electron microscopy. J. Histochem. Cytochem. 24, 933-939. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=182877&dopt=Abstract
Bayer, E. A., Wilchek, M., and Skutelsky, E. (1976) Affinity cytochemistry: The localization of lectin and antibody receptors on erythrocytes via the avidin-biotin complex. FEBS Lett. 68, 240-244.
Skutelsky, E., Danon, D., Wilchek, M., and Bayer, E. A. (1977) Localization of sialyl residues on cell surfaces by affinity cytochemistry. J. Ultrastruct. Res. 61, 325-335.
Kahane, I., Polliack, A., Rachmilewitz, E., Bayer, E. A., and Skutelsky, E. (1978) Distribution of sialic acids on red blood cell membranes in b-thalassaemia. Nature 271, 674-675.
Bayer, E. A., Rivnay, B., and Skutelsky, E. (1979) On the mode of liposome-cell interactions: Biotin-conjugated lipids as ultrastructural probes. Biochim. Biophys. Acta 550, 464-473. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=420828&dopt=Abstract
Bayer, E. A., Zalis, M. G., and Wilchek, M. (1985) 3-(N-Maleimido-propionyl) biocytin: A versatile thiol-specific biotinylating reagent. Anal. Biochem. 149, 529-536. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3935007&dopt=Abstract
Bayer, E. A., Ben-Hur, H., Gitlin, G., and Wilchek, M. (1986) An improved method for the single-step purification of streptavidin. J. Biochem. Biophys. Methods 13, 103-112. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3772022&dopt=Abstract
Wilchek, M., Ben-Hur, H., and Bayer, E. A. (1986) p-Diazobenzoyl biocytin — A new biotinylating reagent for the labeling of tyrosines and histidines in proteins. Biochem. Biophys. Res. Commun. 138, 872-879. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3741438&dopt=Abstract
Bayer, E. A., Safars, M., and Wilchek, M. (1987) Selective labeling of sulfhydryls and disulfides on blot transfers using avidin-biotin technology: Studies on purified proteins and erythrocyte membranes. Anal. Biochem. 161, 262-271. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2437828&dopt=Abstract
Bayer, E. A., Ben-Hur, H., and Wilchek, M. (1988) Biocytin hydrazide — A selective label for sialic acids, galactose and other sugars in glycoconjugates using avidin-biotin technology. Anal. Biochem. 170, 271-281. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2456025&dopt=Abstract
Bayer, E. A., Ben-Hur, H., Hiller, Y., and Wilchek, M. (1989) Postsecretory modifications of streptavidin. Biochem. J. 259, 369-376. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2719654&dopt=Abstract
Bayer, E. A., Grootjans, J. J., Alon, R., and Wilchek, M. (1990) Affinity cleavage and targeted catalysis of proteins using the avidin-biotin system. Biochemistry 29, 11274-11279. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2271711&dopt=Abstract
Bayer, E. A., and Wilchek, M. (1990) Avidin column as a highly efficient and stable alternative for immobilization of ligands for affinity chromatography. J. Molec. Recog. 3, 102-107. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2223160&dopt=Abstract
Reviews
Bayer, E. A. and Wilchek, M. (1978). The avidin-biotin complex as a tool in molecular biology. Trends Biochem. Sci. 3, N237-N239.
Skutelsky, E. and Bayer, E. A. (1979). The ultrastructural localization of cell surface glycoconjugates: Affinity cytochemistry via the avidin-biotin complex. Biol. Cellul. 36, 237-252.
Skutelsky, E., and Bayer, E. A. (1979) Ultrastructural modulations of cell surface determinants in pathological processes. Isr. J. Med. Sci. 15, 675-686.
Bayer, E. A. and Wilchek, M. (1980). The use of the avidin-biotin complex in molecular biology. Methods Biochem. Anal. 26, 1-45.
Wilchek, M. and Bayer, E. A. (1984). The avidin-biotin complex in immunology. Immunol. Today 5, 39-43.
Wilchek, M. and Bayer, E. A. (1988). The avidin-biotin complex in bioanalytical applications. Anal. Biochem. 171, 1-32.
Bayer, E. A., and Wilchek, M. (1990) The application of avidin-biotin technology for affinity-based separations. J. Chromatogr. 510, 3-12. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2205618&dopt=Abstract
Wilchek, M. and Bayer, E. A. (1989). Avidin-biotin technology ten years on: Has it lived up to its expectations? Trends Biochem. Sci. 14, 408-412. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2683260&dopt=Abstract
Bayer, E. A. and Wilchek, M. (1996). The avidin-biotin system. In Immunoassay (E. P. Diamandis and T. K. Christopoulos, ed.), pp. 237-267. Academic Press, San Diego.
Bayer, E. A. and Wilchek, M. (1998). Opera singers, raw eggs and cancer therapy: The avidin-biotin system, nature's gift to molecular biology. Science Spectra 12.
Wilchek, M., Bayer, E. A., and Livnah, O. (2006) Essentials of biorecognition: The (strept)avidin-biotin system as a model for protein-protein and protein-ligand interaction. Immunol. Lett. 103, 27-32.
Engineered Avidins and Streptavidins
The molecular surfaces of both avidin and streptavidin are highlighted by nonspecific or unwanted binding interactions. In order to improve their performance, we have prepared a variety of modified avidins and streptavidins. In this context, we developed an enzymatic (microbial-induced) procedure for the deglycosylation of avidin. The responsible bacterium was isolated from nature and the bacterial strain was identified. In order to neutralize the overall charge, we modified chemically the arginine residues of deglycosylated avidin, and the resultant derivative (which we called "NeutraLite Avidin") was an early example of a commercially successful, bioengineered protein.
We have also identified and isolated two new forms of streptavidin from a different species of Streptomyces. Their genes were cloned and sequenced. More recently, we developed nitrotyrosyl derivatives of both avidin and streptavidin, in which the strong biotin-binding properties of the tetramers can, for the first time, be reversed; we demonstrated several applications of nitro-avidin and nitro-streptavidin for potential use in affinity chromatography, enzyme immobilization and phage-display technology.
References
Bayer, E. A. and Wilchek, M. (1994). Modified avidins for application in avidin-biotin technology: an improvement on nature. In Egg Uses and Processing Technologies (J. S. Sim and S. Nakai, ed.), pp. 158-176. CAB International, Wallingford, UK.
Bayer, E. A., De Meester, F., Kulik, T. and Wilchek, M. (1995). Deglycosylation of egg-white avidin. Appl. Biochem. Biotechnol. 53, 1-9. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7625822&dopt=Abstract
Bayer, E. A., Kulik, T., Adar, R. and Wilchek, M. (1995). Close similarity among streptavidin-like, biotin-binding proteins from Streptomyces. Biochim. Biophys. Acta 1263, 60-66. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7632734&dopt=Abstract
Balass, M., E., M., Bayer, E. A., Fuchs, S., Wilchek, M. and Katchalski-Katzir, E. (1996). Recovery of high-affinity phage from a nitro-streptavidin matrix in phage-display technology. Anal. Biochem. 243, 264-269. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8954559&dopt=Abstract
Morag, E., Bayer, E. A. and Wilchek, M. (1996). Immobilized nitro-avidin and nitro-streptavidin as reusable affinity matrices for application in avidin-biotin technology. Anal. Biochem. 243, 257-263. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8954558&dopt=Abstract
Morag, E., Bayer, E. A. and Wilchek, M. (1996). Reversibility of biotin-binding by selective modification of tyrosine in avidin. Biochem. J. 316, 193-199. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8645205&dopt=Abstract
Pazy, Y., Kulik, T., Bayer, E. A., Wilchek, M., and Livnah, O. (2002) Ligand exchange between proteins. Exchange of biotin and biotin derivatives between avidin and streptavidin. J. Biol. Chem. 277, 30892-30900.
Laitinen, O. H., Nordlund, H. R., Hytonen, V. P., Uotila, S. T., Marttila, A. T., Savolainen, J., Airenne, K. J., Livnah, O., Bayer, E. A., Wilchek, M., and Kulomaa, M. S. (2003) Rational design of an active avidin monomer. J. Biol. Chem. 278, 4010-4014.
Pazy, Y., Eisenberg-Domovich, Y., Laitinen, O. H., Kulomaa, M. S., Bayer, E. A., Wilchek, M., and Livnah, O. (2003) Dimer-tetramer transition between solution and crystalline states of streptavidin and avidin mutants. J. Bacteriol. 185, 4050-4056.
Avidin and Streptavidin Mutants
Despite the availability of crystal structures for both avidin and streptavidin, the precise reasons for the particularly strong interaction with biotin are still not fully understood. The properties of the binding sites of avidin and streptavidin can also be correlated to weaker interactions, since the two proteins both form much weaker associations with the biomimetic dye, 2-(4'-hydroxyazobenzene) benzoic acid (HABA).
Based on the knowledge gained from the structures of avidin and streptavidin, we have designed a variety of avidin mutants. For this work, we established a eukaryotic (baculovirus-insect cell) system together with Prof. Markku Kulomaa (Jyväskylä, Finland). The baculovirus-lepidopteran system was important to our studies, since it yielded viable and soluble recombinant forms of both avidin and streptavidin. We prepared numerous mutations of avidin and streptavidin, in which individual amino acid residues were changed to alter various physicochemical properties of the molecules such as charge, quaternary structure and oligosaccharide side chain. We also exchanged various binding-site residues, which led to alterations in the biotin-binding properties of the resultant mutants. A single mutation, from tryptophan to lysine, at a particularly susceptible conserved position in both proteins (W110K in avidin and W120K in streptavidin yielded dimeric instead of tetrameric molecules, and the biotin-binding property of both mutants was reversible, a long sought-after characteristic. The unusual WK mutations were selected on the basis of their apparent substitutions in an avidin-like domain of an EGF homolog from the sea urchin, called fibropellin.
References
Airenne, K. J., Oker-Blom, C., Marjomäki, V. S., Bayer, E. A., Wilchek, M. and Kulomaa, M. S. (1997). Production of biologically active recombinant avidin in baculovirus-infected insect cells. Protein Expression and Purification 9, 100-108. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9116491&dopt=Abstract
Marttila, A. T., Airenne, K. J., Laitinen, O. H., Bayer, E. A., Wilchek, M. and Kulomaa, M. S. (1998). Engineering of chicken avidin: a progressive series of reduced charge mutants. FEBS Lett. 441, 313-317. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9883906&dopt=Abstract
Laitinen, O. H., Airenne, K. J., Marttila, A. T., Kulik, T., Porkka, E., Bayer, E. A., Wilchek, M. and Kulomaa, M. S. (1999). Mutation of a critical tryptophan to lysine in avidin or streptavidin may explain why sea urchin fibropellin adopts an avidin-like domain. FEBS Lett. 461, 52-58. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10561495&dopt=Abstract
Marttila, A. T., Laitinen, O. H., Airenne, K. J., Kulik, T., Bayer, E. A., Wilchek, M. and Kulomaa, M. S. (2000). Recombinant NeutraLite Avidin: A non-glycosylated, acidic mutant of chicken avidin that exhibits high affinity for biotin and low nonspecific-binding properties. FEBS Lett. 467, 31-36. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10664451&dopt=Abstract
Laitinen, O. H., Marttila, A. T., Airenne, K. J., Kulik, T., Livnah, O., Bayer, E. A., Wilchek, M. and Kulomaa, M. S. (2001). Biotin induces tetramerization of a recombinant monomeric avidin. A model for protein-protein interactions. J. Biol. Chem. 276, 8219-8224. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11076945&dopt=Abstract
Marttila, A. T., Hytönen, V. P., Laitinen, O. H., Bayer, E. A., Wilchek, M., and Kulomaa, M. S. (2003) Mutation of the important Tyr-33 residue of chicken avidin: Functional and structural consequences. Biochem. J. 369, 249-254.
Pazy, Y., Raboy, B., Matto, M., Bayer, E. A., Wilchek, M., and Livnah, O. (2003) Structure-based rational design of streptavidin mutants with pseudo-catalytic activity. J. Biol. Chem. 278, 7131-7134.
Prizant, M., Eisenberg-Domovich, Y., Hytonen, V. P., Kulomaa, M. S., Wilchek, M., Bayer, E. A., and Livnah, O. (2006) Factors dictating the pseudocatalytic efficiency of avidins. J. Mol. Biol. 358, 754-763.
Crystal Structures of Avidins and Streptavidins
Despite the availability of crystal structures for both avidin and streptavidin, the precise reasons for the particularly strong interaction with biotin are still not fully understood. The properties of the binding sites of avidin and streptavidin can also be correlated to weaker interactions, since the two proteins both form much weaker associations with the biomimetic dye, 2-(4'-hydroxyazobenzene) benzoic acid (HABA). Three-dimensional structures for avidin, the avidin-biotin complex and the avidin-HABA complex were elucidated in collaboration with Oded Livnah and Joel Sussman of the Structural Biology Department. More recently, we have concentrated on the crystal structures of avidin and streptavidin mutants as well as the native proteins with other types of biotin derivatives in order to gain further insight into their intriguing molecular characteristics (Figure 3). Continued investigations into the 3D structure of avidin are in collaboration with Oded Livnah (http://sites.huji.ac.il/biolchem/livnah.html)
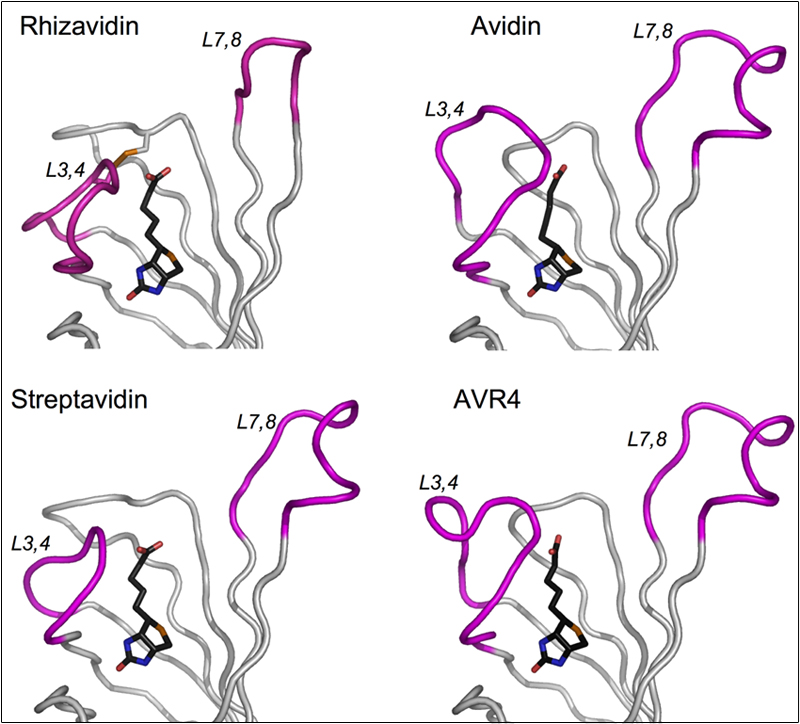
Figure 3. Tube representations of corresponding portions of the rhizavidin, avidin, streptavidin, and AVR4 monomers. The biotin molecules are shown in black and the L3,4 and L7,8 loops are indicated in magenta. Substantial differences in the L3,4 loop conformations between the four molecules are observed, dictated mainly by their amino acid composition.
References
Livnah, O., Bayer, E. A., Wilchek, M., and Sussman, J. L. (1993) Structure of the complex between avidin and the dye, 2-(4'-hydroxyazobenzene) benzoic acid (HABA). FEBS Lett. 328, 165-168. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8344421&dopt=Abstract
Livnah, O., Bayer, E. A., Wilchek, M., and Sussman, J. L. (1993) Three-dimensional structures of avidin and the avidin-biotin complex. Proc. Natl. Acad. Sci. USA 90, 5076-5080. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8506353&dopt=Abstract
Laitinen, O. H., Marttila, A. T., Airenne, K. J., Kulik, T., Livnah, O., Bayer, E. A., Wilchek, M., and Kulomaa, M. S. (2001) Biotin induces tetramerization of a recombinant monomeric avidin. A model for protein-protein interactions. J. Biol. Chem. 276, 8219-8224. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11076945&dopt=Abstract
Huberman, T., Eisenberg-Domovich, Y., Gitlin, G., Kulik, T., Bayer, E. A., Wilchek, M., and Livnah, O. (2001) Chicken avidin exhibits pseudo-catalytic properties: Biochemical, structural and electrostatic consequences. J. Biol. Chem. 276, 32031-32039. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11395489&dopt=Abstract
Pazy, Y., Laitinen, O. H., Ravoy, B., Kulomaa, M. S., Wilchek, M., Bayer, E. A., and Livnah, O. (2001) Crystallization and preliminary X-ray analysis of W120K mutant of streptavidin. Acta Crystallogr. D 57, 1885-1886. http://www.ncbi.nlm.nih.gov:80/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11717505&dopt=Abstract
Eisenberg-Domovich, Y., Hytönen, V. P., Kulomaa, M. S., Wilchek, M., Bayer, E. A., and Livnah, O. (2005) Crystal structure of an avidin-related protein: Insight into high-affinity biotin binding and protein stability. J. Biol. Chem. 61, 528-538.
Hayouka, R., Eisenberg-Domovich, Y., Hytönen, V. P., Määttä, J. A. E., Nordlund, H. R., Kulomaa, M. S., Wilchek, M., Bayer, E. A., and Livnah, O. (2008) Critical importance of loop conformation to avidin-enhanced hydrolysis of an active biotin ester. Acta Crystallogr., 302-308.



