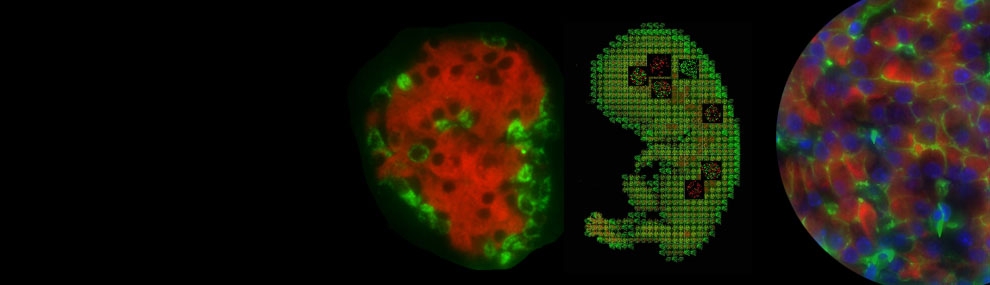
Genes of the FoxA family are expressed at an early stage of mouse development during the formation of definitive endoderm. The first to be activated is FoxA2, initially in the anterior primitive streak and the node at embryonic day 6.5 in the mouse. FoxA2 (together with family member FoxA1) is required for proper development of endoderm-derived organs such as liver, lung and pancreas, and is thus considered a master regulator of early endoderm formation and endoderm lineage establishment.
FoxA2 has been described as a “pioneer factor” that binds to the chromatin of a progenitor cell prior to target gene activation along with factors that help to modify the chromatin upon gene activation during cellular differentiation. FoxA2 is also expressed later in development, in endoderm-derived tissues, including liver, lung, stomach, pancreas, small intestine, and colon. In pancreatic islets, FoxA2 is a direct activator of Pdx1, which places it near the top of the pancreatic transcription factor hierarchy. Currently, little information is available regarding the precise molecular mechanisms that control the activation of this gene during different stages of development.
In this study, we report that a CpG island in the promoter region of the FoxA2 gene paradoxically shows high levels of DNA methylation in endoderm lineage FoxA2-expressing tissues and low levels in non-expressing tissues. Using reporter gene assay, this CpG island region was found to inhibit reporter gene expression, an effect that was abolished by in vitro methylation of the inhibitory fragment. Furthermore, using a ChIP assay, we observed that this region is bound by the Polycomb group protein SUZ12 and the DNA methyltransferase DNMT3b in hESCs, where this region is unmethylated; this binding is accompanied by high levels of H3K27me3. Furthermore, inhibition of methylation by 5-aza-2'-deoxycytidine (5-aza-dC) or directed DNMT3b knockdown led to decreased activation of FoxA2 during stem cell differentiation toward endoderm progenitors. These results indicate that methylation of the CpG island plays a key role in FoxA2 gene activation in both early and late stages of FoxA2 expression.
Based on these results, we hypothesize that in hESCs, a lack of DNA methylation permits binding of a sequence-specific repressor protein that mediates the binding to this region of SUZ12, a component of PRC2 that includes also the protein EED and EZH2. This protein directly methylates H3K27, a modification that also contributes to the repression of the gene in hESC. SUZ12 in turn recruits DNMT3b. We propose that upon differentiation, DNMT activation leads to CpG island methylation, which in turn causes loss of repressor protein binding.
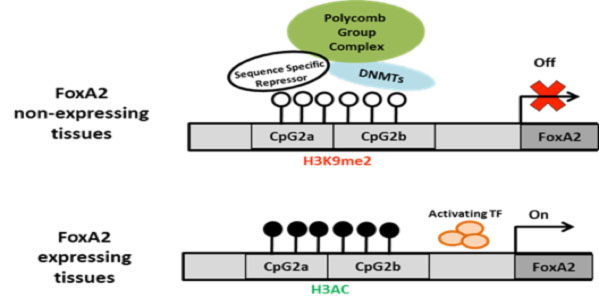
mechanism of activation of FoxA2 gene expression during development. Top: schematic illustration of the FoxA2 activation model proposed. CpG dinucleotides are presented as white (unmethylated ) and black (methylated), Polycomb repressive complex, DNMT and postulated sequence specific repressor protein are indicated.