Insulin is a key metabolic hormone, essential for maintenance of normal levels of blood glucose. Insulin is produced exclusively by beta cells located within islets of the pancreas. Insufficient secretion of insulin leads to diabetes and its hallmark symptom of chronic elevated blood glucose levels. In type 1 diabetes, autoimmune destruction of the beta cells leads to destruction of virtually all beta cells. Treatment involves regular injections of insulin.
In type 2 diabetes, typically obesity leads to insulin resistance and a resulting need for increased insulin production. This in turn can lead to beta cell stress (“exhaustion”) and dysfunction. In early stages of the disease, oral drugs are effective in restoring normoglycemia. However in many cases, the disease progresses to a stage where insulin treatment is required to maintain normoglycemia. The factors linking obesity /insulin resistance to impaired beta cell function /diabetes are not clear. Chronic exposure to elevated glucose levels (glucotoxicity) chronic lipids (lipotoxicity) and beta cell stress have all be suggested as possibly leading to beta cell dysfunction.
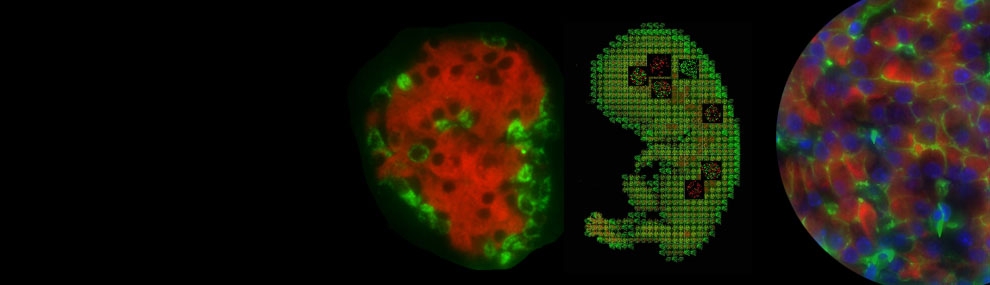
GPR40 (FFAR1), and GPR41 (FFAR3), are members of a small family of G protein-coupled receptors GPCRs; they are both expressed preferentially in beta cells. GPR40 is a receptor for LCFAs, and GPR41 a receptor for SCFAs. We previously found that the GPR41 gene is transcribed in β cells in a unique pattern as a bicistronic mRNA together with the upstream gene GPR40 and that translation of GPR41 mRNA is mediated via an internal ribosome entry site element located in the intergenic region. Preferred ligands of GPR40 are LCFAs and of GPR41 SCFAs. Knockout of GPR40 in mice or knock-down in cultured beta cells leads to attenuation of LCFA-dependent insulin secretion, thus this receptor is an important component in conferring responsiveness of β cells to LCFAs.
To examine the possible role of SCFAs in function of pancreatic beta cells, we analyzed mouse models of gain and loss of function of the GPR41 (FFAR3) gene GPR41 gain of function (41 Tg) and GPR41 loss of function (KO 41), resulted in complementary changes in glucose tolerance, without significant effects on insulin sensitivity. The KO 41 mice also showed fasting hypoglycemia, consistent with increased basal insulin secretion in vitro. Mirroring this, 41 Tg islets secreted less insulin in vitro. Microarray analysis of islets from 41 Tg mice indicated significant alterations in gene expression patterns; a number of these changes were also observed upon direct incubation of the SCFA propionate with cultured beta cells and islets, in a GPR41-dependent pathway. Taken together, our results indicate that SCFAs, acting through GPR41, may play an important role in fine-tuning of insulin secretion in fed and fasted states.
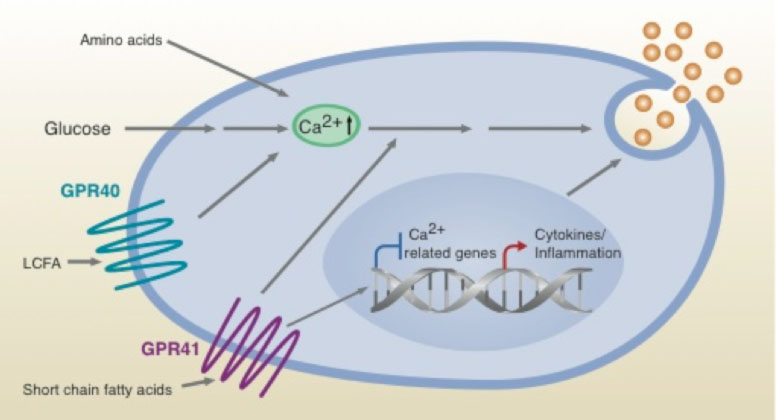
Scheme illustrating proposed effects on beta cell insulin secretion of SCFAs via GPR41, LCFAs via GPR40 and additional secretagogues