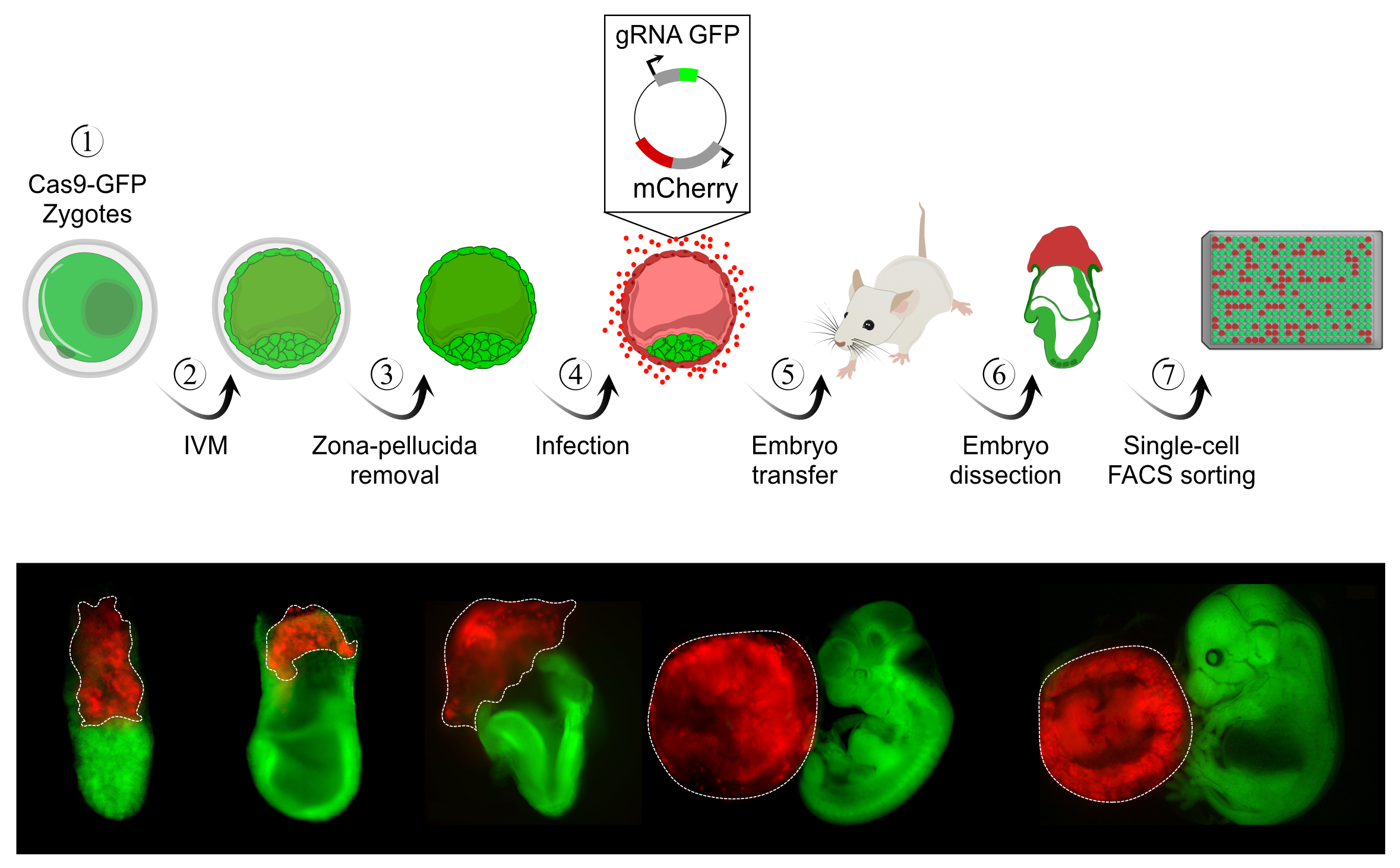
Understanding how extrinsic signals from the immediate 3D environment affect cellular commitment represent a major open challenge in the field, requiring synthesizing ideas and expertise from several disciplines. To this end, we are developing new methodologies for indexing spatial epigenomics from single embryonic cells with the aim of constructing highly quantitative and relatable spatiotemporal models of gastrulation. To study how embryonic niches are formed within a dynamically changing spatial environment, we focus on one of the most fundamental lineage decisions in mammals - the specification of germ cells in the early embryo. The induction of primordial germ cells in a confined spatial localization suggests the involvement of tightly regulated extrinsic cues that we are working to unravel using computational modeling, perturbation systems, and combinatorial cell marking at the DNA, RNA, and protein levels. Finally, one of our favorite paradigms for inter-lineage interactions is the cross-talk between the placenta and the embryo, which we study using systems we develop for controlled lineage manipulation in vivo with robust readout from single cells (see below).