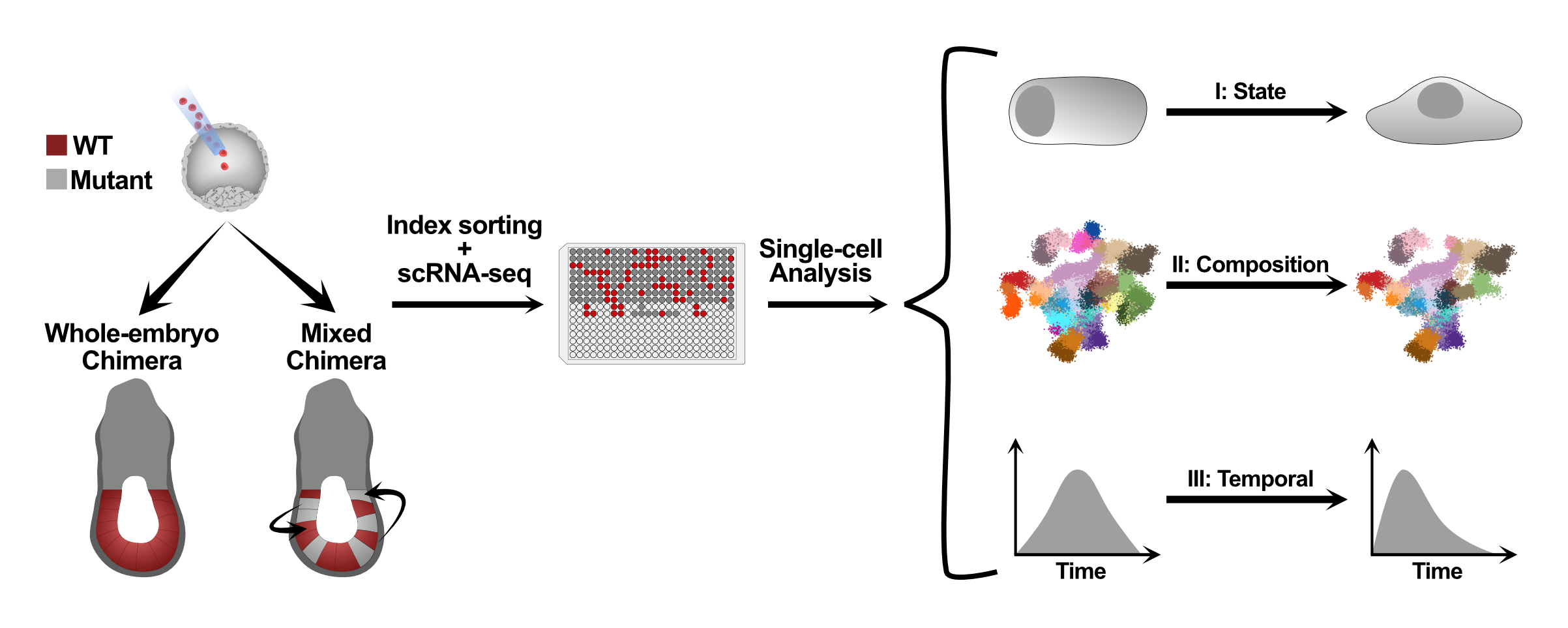
A major effort in our lab is devoted to dissecting epigenetic mechanisms that provide embryonic cells with robust identities. This is addressed by combining state-of-the-art single cell epigenomics, flexible reporter systems we develop, and time-resolved atlases of mutant embryos obtained via classical chimera assays (see below scheme). In this manner, we have recently demonstrated how embryonic atlases can move from descriptive tools into a revolution in our ability to understand the intra-cellular function of epigenetic regulators.
In the coming years, we are determined to engage the formidable challenge of cracking the in-cis code and specificity of trans-acting factors that together orchestrate cellular decisions in the embryo.
Germline reprogramming and epigenetic memory
In mammals, epigenetic reprogramming following fertilization and during specification of the germline robustly prevents erroneous transmission of epigenetic information between generations. We are fascinated by these processes and their broad implications for other fields such as regenerative medicine, cancer, and aging. Mammalian parental imprinting is another active research area in the lab. In this remarkable epigenetic paradigm, few gamete-specific DNA methylation marks escape global remodeling after fertilization resulting in the parent-specific monoallelic expression of imprinted genes. We are working to elucidate mechanisms controlling this truly exquisite form of gene expression regulation, with the aim of understanding more globally how epigenetic information is transmitted and maintained throughout cell replications.