We have previously demonstrated that Runx3-/- mice spontaneously develop inflammatory bowel disease (IBD). Runx3 is expressed in resident intraepithelial CD8+ T cells and in the lamina propria (LP) dendritic cells (DCs) and macrophages, but not in gastrointestinal tract (GIT) epithelium, indicating that loss of leukocytic cell-autonomous functions of Runx3 is the underlying cause of Runx3-/- IBD.
Adoptive transfer of Runx3-/- fetal liver cells to wild type (WT) irradiated recipient mice resulted in spontaneous development of IBD demonstrating unequivocally the involvement of Runx3-/- leukocytes in IBD ethiology. Runx3-/- mice also develop an asthma like phenotype due to hyperactivated lung DCs, further suggesting that Runx3 expression in mononuclear phagocytes (MNP) is an essential component of mucosal immunity.
The aim of this project is to elucidate the contribution of Runx3-/- DCs and macrophages to IBD development. To this end we use Runx3f/f mice generated in our lab as a mouse model in which Runx3 is specifically ablated in DCs and macrophages (DC-macrophageRunx3-/- mice) (Figure 1). Currently we are using CyTOF (Figure 2) to characterize GIT LP MNP and other hematopoietic cell types, during IBD, to gain insight into the mechanisms of IBD generation due to the absence of Runx3 in MNPs.
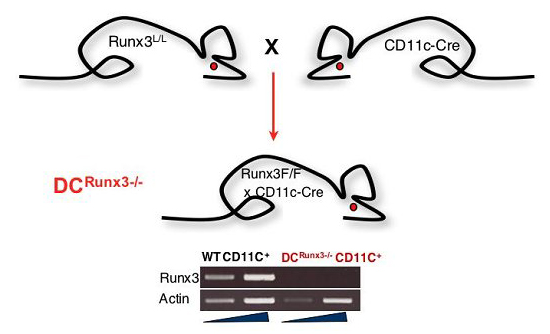
Figure 1 Generation of DC-macrophageRunx3-/- mice by crossing Runx3F/F mice onto transgenic CD11c-Cre mice. The Cre gene regulated by the CD11c promoter mediates specific inactivation of Runx3 in DC and macrophages. Runx3 RT-PCR of spleen sorted CD11c+ DC RNA shows complete loss of Runx3 expression in the DC-macrophageRunx3-/-.
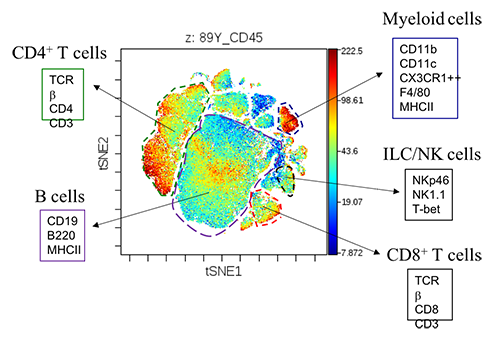
Figure 2 CyTOF analysis of WT colon LP CD45+ cells.