Autophagy-dependent cell death (ADCD)
Autophagy is a survival mechanism that often counters other death pathways. In some circumstances, however, it can be used as a death pathway, in particular in lower organisms during development. We have shown that hyper-activation of autophagy can likewise lead to cell death in human cells, and have characterized the process and its mechanism.
- Using resveratrol treated lung cancer cells as a model for ADCD, we have characterized a unique morphology that is consistent with over-consumption of cellular components leading to catastrophic internal me
 mbrane failure.
mbrane failure.
EM showing accumulation of autophagic vacuoles (AV) in A549 lung cancer cells treated with resveratrol (RSV) to induce ADCD. From Dasari, et al, 2017, Cell Death Differ 24:1288-1302.
- In a signalome-wide siRNA screen to identify genes that were necessary for resveratrol-induced death, we uncovered a novel pathway involving GBA1, a lysosomal enzyme involved in ceramide metabolism, which in mutant form, leads to Gaucher Disease.
- The physiologic relevance of GBA1 for ADCD was established during Drosophila metamorphosis. Mitgut regression of larvae, which is known to be mediated by autophagy-dependent cell elimination, was delayed upon knock-down of the fly GBA1 orthologue.
Cell Extrusion in mouse embryo
Cell death is an important component of early embryonic development, necessary for maintaining cell number and sculpting the embryo. Much of this cell death is apoptosis, yet mouse embryos in which key apoptosis genes are deleted undergo normal development during these early stages. Based on this, we hypothesize that alternative death pathways compensate and/or function in parallel to apoptosis. We specifically addressed this during the cavitation process in the post-implantation mouse embryo. Using a combination of knock-out mice, in situ whole-mount embryos, and EM, we discovered a terminal cell fate in which apical cells extrude portions of their cellular content, including nuclei and other organelles, into the lumen. This novel process of cell shedding occurs independently of caspases, thus representing an alternative means of eliminating cells in the early embryo.
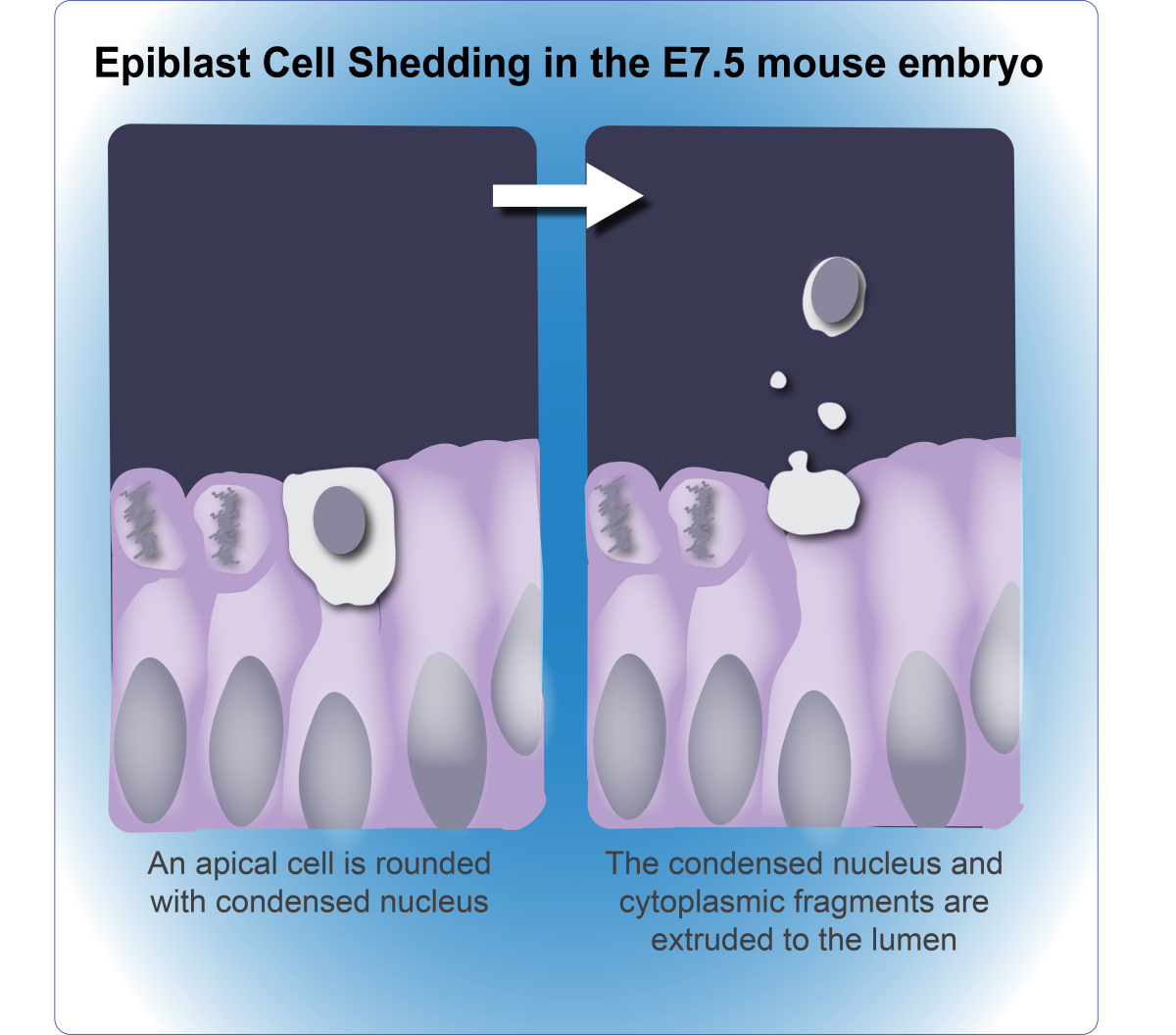
From Halimi, et al. 2022 Cell Death Differ. 29:6.
Integrating radiation-induced senescence into the PCD network
Senescence and cell death are alternative cell fates that are often triggered by the same stressor, and molecular switches can control whether one or the other is activated. Senescence can thus be considered an annex of the PCD network mediating the balance between life and death of the cell, and is likely to share common molecular regulators. To identify such dual-function regulators, we developed a model of radiation-induced senescence in cultured lung carcinoma cells, and using a cell death siRNA library, we screened for PCD proteins that block radiation-induced senescence. In this manner we identified mitochondrial serine protease HtrA2, which is necessary for the continuous maintenance of proliferation arrest, and by cleaving the intermediate filament protein vimentin, is involved in the cytoskeletal reorganization that accompanies radiation-induced senescence.
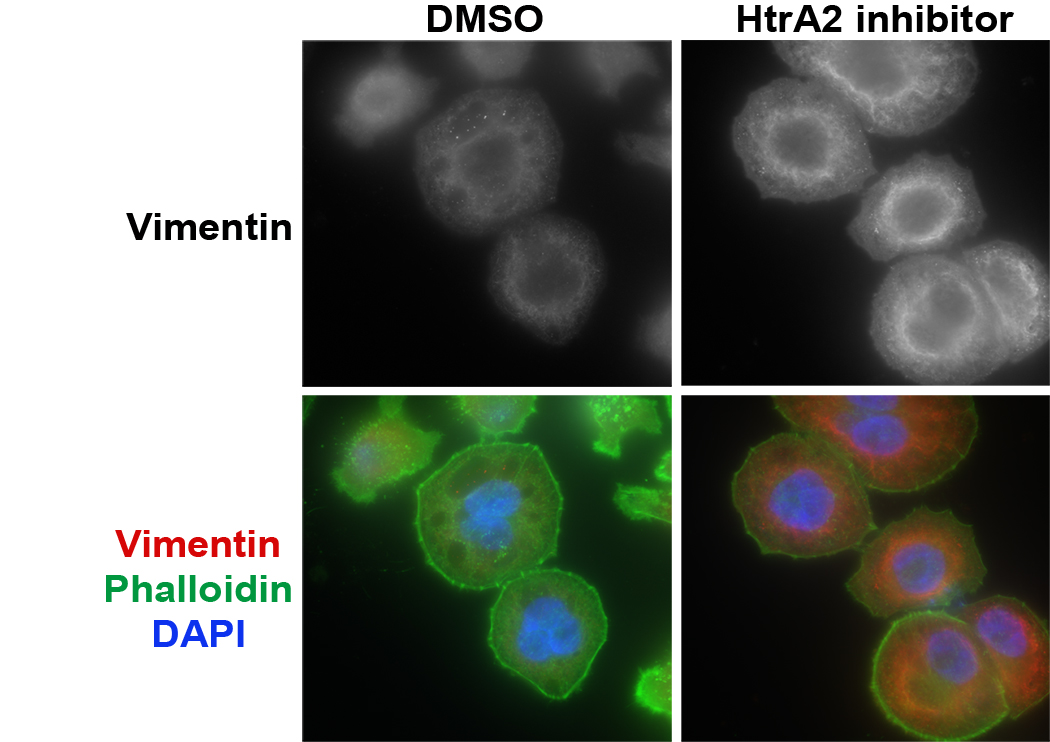
NCI-H460 cells were irradiated and stained for vimentin (red) and actin cytoskeleton (green, phallodin). Treatment with HtrA2 inhibitor restores the vimentin cytoskeletal network that collapses following irradiation. From Hammer, at al., 2022. Mol Oncol. 16:1365-1583.


