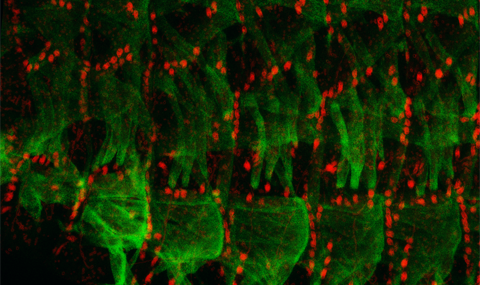
The shape and morphology of nuclei in differentiated tissues is considered as a major epigenetic factor affecting gene expression, chromatin organization and signaling pathways. It is believed that differentiated cells acquire a typical nuclear architecture allowing tissue-specific gene expression required for function. Muscle fiber growth responds rapidly to muscle use, or disuse, and our major hypothesis is that this response depends primarily on the cytoplasmic forces applied on the nuclei during enhanced or reduced muscle activity. We showed recently that proteins of the LINC complex capable of transducing mechanical forces between the cytoplasm and the nucleus participate in the nuclear signaling response to cytoplasmic strain (Elhanany-Tamir er al., 2012, Volk, 2013, Wang et al., 2015). Our research is aimed to (a) Identify the molecular components involved in the maintenance of muscle nuclear shape. (b) Reveal the contribution of muscle nuclear morphology to muscle activity, and (c) Identify the molecular pathway involved in translating muscle activity into the muscle cellular response.
The Development and Cell biology of Striated Muscles