Cancer
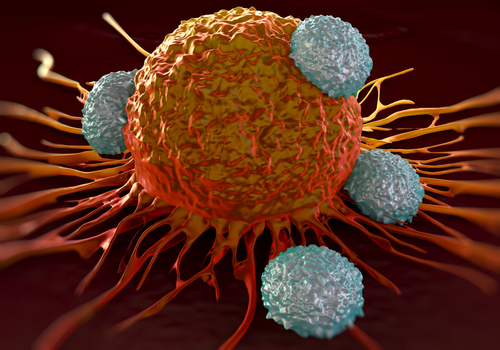 Understanding epiProteome regulation in cancer is critical for uncovering how dynamic post-translational modifications (PTMs) and proteasomal degradation shape tumor progression, immune evasion, and therapy resistance. Beyond PTMs, the selective degradation of proteins by the ubiquitin-proteasome system (UPS) and immunoproteasome dictates the stability of key oncogenic and tumor-suppressive factors, modulating cellular signaling, antigen presentation, and stress responses. Unlike static genetic and transcriptomic landscapes, the epiProteome represents the functional proteomic layer that integrates environmental cues, metabolic shifts, and proteostasis dynamics to shape cancer cell fate and tumor microenvironment interactions. By leveraging cutting-edge mass spectrometry, computational modeling, and functional assays, our lab deciphers how PTMs and proteasomal degradation rewire oncogenic networks, influence immune recognition, and drive tumor heterogeneity. This knowledge not only provides novel biomarkers for precision oncology but also uncovers actionable vulnerabilities that can be exploited for next-generation cancer therapies.
Understanding epiProteome regulation in cancer is critical for uncovering how dynamic post-translational modifications (PTMs) and proteasomal degradation shape tumor progression, immune evasion, and therapy resistance. Beyond PTMs, the selective degradation of proteins by the ubiquitin-proteasome system (UPS) and immunoproteasome dictates the stability of key oncogenic and tumor-suppressive factors, modulating cellular signaling, antigen presentation, and stress responses. Unlike static genetic and transcriptomic landscapes, the epiProteome represents the functional proteomic layer that integrates environmental cues, metabolic shifts, and proteostasis dynamics to shape cancer cell fate and tumor microenvironment interactions. By leveraging cutting-edge mass spectrometry, computational modeling, and functional assays, our lab deciphers how PTMs and proteasomal degradation rewire oncogenic networks, influence immune recognition, and drive tumor heterogeneity. This knowledge not only provides novel biomarkers for precision oncology but also uncovers actionable vulnerabilities that can be exploited for next-generation cancer therapies.
Neurodegeneration
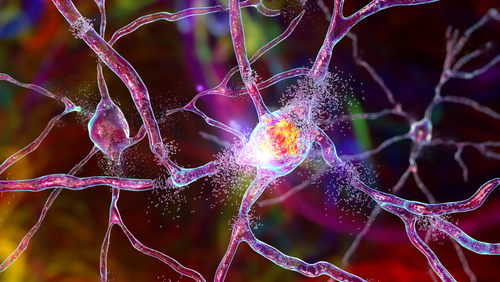 The ubiquitin system plays a pivotal role in maintaining proteostasis, and E3 ligases serve as key regulators of protein turnover, shaping cellular responses across different tissues and disease states. In the context of neurodegeneration, where disrupted protein homeostasis leads to toxic protein accumulation, examining E3 ligase activity across human tissues provides critical insights into how ubiquitin signaling governs neuronal survival, stress responses, and degradation pathways. Understanding how diverse cellular signals regulate E3 ligase function can uncover fundamental mechanisms of epiProteome regulation and identify novel therapeutic targets for neurodegenerative disorders.
The ubiquitin system plays a pivotal role in maintaining proteostasis, and E3 ligases serve as key regulators of protein turnover, shaping cellular responses across different tissues and disease states. In the context of neurodegeneration, where disrupted protein homeostasis leads to toxic protein accumulation, examining E3 ligase activity across human tissues provides critical insights into how ubiquitin signaling governs neuronal survival, stress responses, and degradation pathways. Understanding how diverse cellular signals regulate E3 ligase function can uncover fundamental mechanisms of epiProteome regulation and identify novel therapeutic targets for neurodegenerative disorders.
Infectious diseases
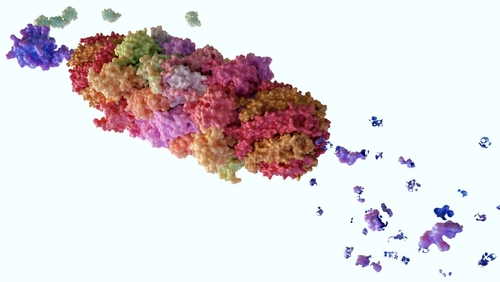 Our lab has uncovered a previously unknown immune mechanism, revealing that proteasomal degradation actively generates antimicrobial peptides, forming a natural defense system against bacterial infections. This discovery challenges long-standing views of the proteasome as a mere “cellular garbage can” and establishes its role as a key player in cell-autonomous innate immunity. By identifying hundreds of thousands of Proteasome-Derived Defense Peptides (PDDPs) with activity against both gram-positive and gram-negative bacteria, we open a new avenue for combating antibiotic resistance. However, many questions remain to be addressed: What is the sensing mechanism for PDDP production? How are they secreted, and how are they regulated across different tissues in immune and non-immune cells? How is this mechanism altered upon infection by different pathogens? We strive to uncover new insights into proteolysis-driven immunity.
Our lab has uncovered a previously unknown immune mechanism, revealing that proteasomal degradation actively generates antimicrobial peptides, forming a natural defense system against bacterial infections. This discovery challenges long-standing views of the proteasome as a mere “cellular garbage can” and establishes its role as a key player in cell-autonomous innate immunity. By identifying hundreds of thousands of Proteasome-Derived Defense Peptides (PDDPs) with activity against both gram-positive and gram-negative bacteria, we open a new avenue for combating antibiotic resistance. However, many questions remain to be addressed: What is the sensing mechanism for PDDP production? How are they secreted, and how are they regulated across different tissues in immune and non-immune cells? How is this mechanism altered upon infection by different pathogens? We strive to uncover new insights into proteolysis-driven immunity.
Metabolism
 Our lab is investigating the critical role of E3 ligases in regulating metabolic and insulin signaling pathways. E3 ligases, which control the ubiquitination of target proteins, are integral to maintaining cellular homeostasis and response to metabolic changes. We aim to uncover how specific E3 ligases regulate key players in insulin signaling and metabolic pathways, shedding light on their involvement in diseases such as diabetes, obesity, and metabolic syndrome. By understanding the precise mechanisms through which E3 ligases modulate these pathways, we hope to identify novel therapeutic targets for improving metabolic health and combating insulin resistance.
Our lab is investigating the critical role of E3 ligases in regulating metabolic and insulin signaling pathways. E3 ligases, which control the ubiquitination of target proteins, are integral to maintaining cellular homeostasis and response to metabolic changes. We aim to uncover how specific E3 ligases regulate key players in insulin signaling and metabolic pathways, shedding light on their involvement in diseases such as diabetes, obesity, and metabolic syndrome. By understanding the precise mechanisms through which E3 ligases modulate these pathways, we hope to identify novel therapeutic targets for improving metabolic health and combating insulin resistance.
