Endoplasmic reticulum (ER) stress and major chemotherapeutic agents damage DNA by generating reactive oxygen species (ROS). We found that ER stress and major chemotherapeutic agents induce leukotriene C4 (LTC4) biosynthesis in cells of non-hematopoietic lineage. Following ER stress or chemotherapy, an LTC4 biosynthetic machinery, consisting of microsomal glutathione-S-transferase 2 (MGST2), 5-lipoxygenase (5-LO), 5-lipoxygenase-activating protein (FLAP) and cytoplasmic phospholipase A2 (cPLA2), was activated by assembly at the nuclear envelope. ER stress and chemotherapy also triggered nuclear translocation of the two LTC4 receptors, CysLTR1 & CysLTR2. Acting in an intracrine manner, LTC4 then elicits nuclear translocation of NADPH oxidase 4 (NOX4), resulting in nuclear ROS accumulation, oxidative DNA damage and dsDNA breaks. Mgst2 deficiency, RNAi and LTC4 receptor antagonists abolished ER stress- and chemotherapy-induced ROS accumulation and DNA damage in vitro and in mouse kidneys. Cell death and mouse morbidity were also significantly attenuated. Hence, MGST2-generated LTC4 is the major mediator of ER stress- and chemotherapy-triggered oxidative stress and oxidative DNA damage, thereby augmenting cell death. Tumor cells of hematopoietic origin do not express MGST2. Indeed, they remained refractive to chemotherapy in the presence of LTC4 inhibitors. We therefore propose that LTC4 inhibitors, commonly used for the treatment of asthma, may alleviate chemotherapy-associated morbidities when used in hematologic malignancies. Our findings provide the mechanism by which these asthma drugs attenuated the neurotoxicity of amyloid β peptide, damage to heart myocytes by hypoxia-reperfusion and gentamycin-triggered kidney damage, as previously reported by several other research teams. Furthermore, NOX4 has been implicated in other major human pathologies, including metabolic diseases, neurodegeneration and osteoporosis. Therefore, inhibition of its activation by LTC4 receptor antagonists, already serving as approved asthma drugs, may be of even a broader clinical significance.
The following scheme summarises the MGST2-LTC4 mode of action:
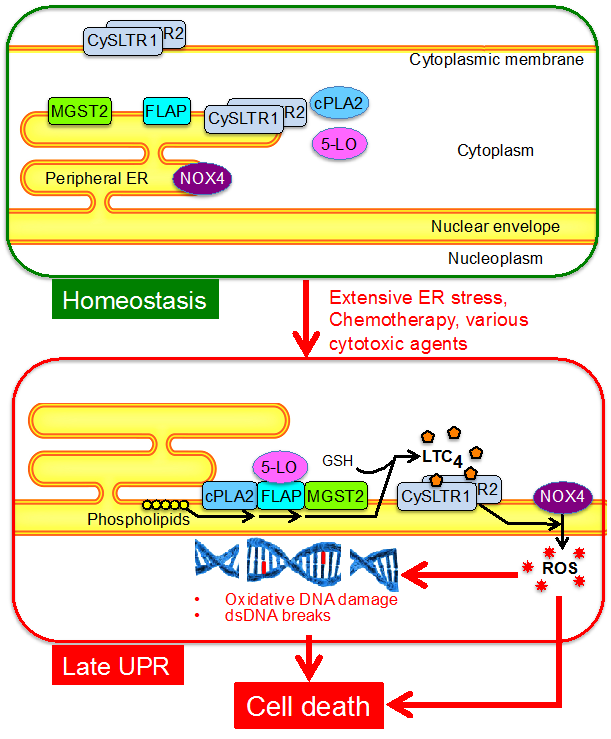
Scheme: The stress-triggered signalling pathway leading to DNA damage and cell death.
Glossary: LTC4, leukotriene C4; CysLTR1 & CysLTR2, the two LTC4 receptors; MGST2, microsomal glutathione S transferase 2; FLAP, 5-LO activating protein; cPLA2, cytoplasmic phospholipase A2; 5-LO, 5-lipoxygenase; ER, endoplasmic reticulum; NOX4, NADPH oxidase 4; ROS, reactive oxygen species.

