Our group is currently studying the adaptation of tumor cells to endoplasmic reticulum (ER) stress. Nutrient shortage, hypoxia and accumulation of toxic metabolites due to limited vasculature trigger continuous ER stress in solid tumors. Radiation, chemotherapy and specifically proteasome inhibitors exert their anti-tumor activity at least in part by further increasing the tumor ER stress. In order to survive, tumor cells adapt to continuous ER stress but the mechanisms that mediate this adaptation are poorly characterized.
We found that tumor cells adapt to continuous ER stress by regulating the expression of the transcription factor C/EBP-ß. Over-expression of C/EBP-ß was reported in many tumor types, including breast cancer, colon cancer, melanoma and more but its exact mode of action as tumor promoters was not known. Our in vivo and in vitro studies show that C/EBP-ß promotes tumor progression by attenuating ER stress-triggered cell death (see Figs. 1 &2).
Currently, we are characterizing C/EBP-ß-induced genes, asking how do they contribute to tumor cell survival and to drug resistance. We hope that such studies will eventually lead to development of improved therapeutic approaches to cancer.
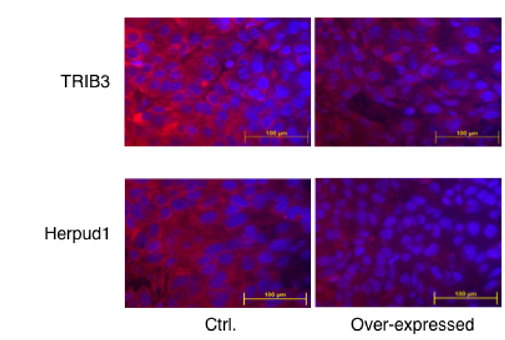
Figure 1. Attenuation of ER stress in a melanoma tumor. Over-expression of C/EBP-ß reduced tumor ER stress, as determined by immunostaining of the two of ER stress markers – TRIB3 and Herpud1 (red). cell nucleai were counter-stained with DAPI.
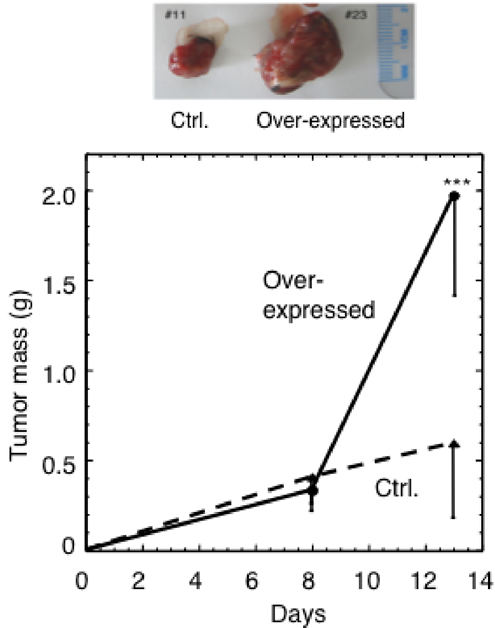
Figure 2. Attenuation of ER stress led to a fourfold increase of the average tumor mass. Mice were inoculated with melanoma tumor cells. C/EBP-ß was induced and tumor mass was determined after 8 and 13 days. Upon attenuation of the tumor ER stress a statistically significant increase in the average tumor mass (P<0.0001; N=9 per group) was observed.

